
ALEKS 360 ACCESS CARD F/GEN. ORG.CHEM
3rd Edition
ISBN: 9781264452545
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Question
Chapter 2.1, Problem 2.7P
Interpretation Introduction
Interpretation:
The elements in the ball and stick model of halothane should be identified.
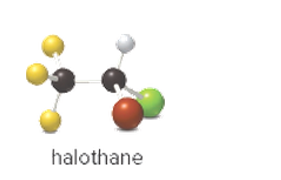
Concept Introduction:
Organic compounds are the compounds which are mainly composed C and H atoms. The branch of chemistry that deals with preparation, reactions, and properties of organic compounds is said to be
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
If I have 30% H2O2, indicate how to prepare a 6% H2O2 solution.
7)
8)
FCI II
-C-C-C=C-C
||
Br Br
||
-C=C-Br
-CEC-C-C-
10)
11)
F Br
i
OH
مله
12)
Br
i
13)
14)
15)
CH3CHFCHFC=CH
C(OH)Br2CHF(CH2)4CH2CH3
CH3(CH2)3CH=CH(CH2)2CH3
Name
1) 3-fluoro, 1-butene
2) 2-heptene
2,3-difluoro-
1-pentene
4) 6-iodo,4-methyl-
2-decyne
5) 4,4-dibromo-
1,2-butandiol
Complete structural formula
F
-C=C-C-C-
Line formula
Condensed structural formula
N
F
CH2=CHCHFCH3
Chapter 2 Solutions
ALEKS 360 ACCESS CARD F/GEN. ORG.CHEM
Ch. 2.1 - Give the symbol for each element. a. calcium, a...Ch. 2.1 - Give the name corresponding to each element...Ch. 2.1 - Locate each element in the periodic table and...Ch. 2.1 - Classify each micronutrient in Figure 2.2 as a...Ch. 2.1 - Identify the elements used ineach example of...Ch. 2.1 - Identify the elements in each chemical formula,...Ch. 2.1 - Prob. 2.7PCh. 2.2 - Prob. 2.8PCh. 2.2 - Prob. 2.9PCh. 2.2 - For the given atom: (a) determine the number of...
Ch. 2.2 - How many protons, neutrons, and electrons are...Ch. 2.2 - What is the mass number of an atom that contains...Ch. 2.3 - For each atom give the following information: [1]...Ch. 2.3 - Magnesium has three isotopes that contain 12, 13,...Ch. 2.3 - Prob. 2.15PCh. 2.3 - Calculate the atomic weight of each element given...Ch. 2.4 - Prob. 2.17PCh. 2.4 - Prob. 2.18PCh. 2.4 - Identify the element fitting each description. an...Ch. 2.4 - Identify each highlighted element in the periodic...Ch. 2.5 - How many electrons are present in each shell,...Ch. 2.6 - What element has each electronic configuration? a....Ch. 2.6 - What element(s) in the first and second period fit...Ch. 2.6 - Draw an orbital diagram for each element; (a)...Ch. 2.6 - Give the electronic configuration for each element...Ch. 2.7 - Prob. 2.26PCh. 2.7 - Determine the number of valence electrons and give...Ch. 2.7 - Prob. 2.28PCh. 2.7 - Give the electron-dot symbol for each element: (a)...Ch. 2.8 - Which element in each pair has the larger atomic...Ch. 2.8 - Prob. 2.31PCh. 2.8 - (a) Which of the indicated atoms has the smaller...Ch. 2 - Identify the elements used in each example of...Ch. 2 - Write a chemical formula for each example of...Ch. 2 - Give the name of the elements in each group of...Ch. 2 - What element(s) are designated by each symbol or...Ch. 2 - Does each chemical formula represent an element or...Ch. 2 - Identify the elements in each chemical formula and...Ch. 2 - Prob. 2.39PCh. 2 - Prob. 2.40PCh. 2 - Give all of the terms that apply to each...Ch. 2 - Give all of the terms that apply to each...Ch. 2 - Give the following information about the atom...Ch. 2 - Give the following information about the atom...Ch. 2 - Prob. 2.45PCh. 2 - Prob. 2.46PCh. 2 - Label each region on the periodic table. Noble...Ch. 2 - Identify each highlighted element in the periodic...Ch. 2 - Prob. 2.49PCh. 2 - Prob. 2.50PCh. 2 - Prob. 2.51PCh. 2 - Prob. 2.52PCh. 2 - Prob. 2.53PCh. 2 - Prob. 2.54PCh. 2 - Prob. 2.55PCh. 2 - Prob. 2.56PCh. 2 - How many protons, neutrons, and electrons are...Ch. 2 - Give the number of protons, neutrons, and...Ch. 2 - Write the element symbol that fits each...Ch. 2 - Write the element symbol that fits each...Ch. 2 - Calculate the atomic weight of silver, which has...Ch. 2 - Calculate the atomic weight of antimony, which has...Ch. 2 - Prob. 2.63PCh. 2 - What is the maximum number of electrons that can...Ch. 2 - Prob. 2.65PCh. 2 - Use an orbital diagram to write the electronic...Ch. 2 - Prob. 2.67PCh. 2 - For each element in Problem 2.66: Write out the...Ch. 2 - Prob. 2.69PCh. 2 - Prob. 2.70PCh. 2 - Give the total number of electrons, the number of...Ch. 2 - Give the total number of electrons, the number of...Ch. 2 - Prob. 2.73PCh. 2 - Prob. 2.74PCh. 2 - Prob. 2.75PCh. 2 - Prob. 2.76PCh. 2 - Prob. 2.77PCh. 2 - Prob. 2.78PCh. 2 - Prob. 2.79PCh. 2 - Prob. 2.80PCh. 2 - Prob. 2.81PCh. 2 - Prob. 2.82PCh. 2 - Prob. 2.83PCh. 2 - Prob. 2.84PCh. 2 - Prob. 2.85PCh. 2 - Prob. 2.86PCh. 2 - Prob. 2.87PCh. 2 - Prob. 2.88PCh. 2 - Rank the atoms in each group in order of...Ch. 2 - Prob. 2.90PCh. 2 - Prob. 2.91PCh. 2 - Prob. 2.92PCh. 2 - Prob. 2.93PCh. 2 - Prob. 2.94PCh. 2 - Prob. 2.95PCh. 2 - (a) What is the chemical formula for...Ch. 2 - Answer the following questions about the...Ch. 2 - Platinum is a precious metal used in a wide...Ch. 2 - Prob. 2.99PCh. 2 - Answer the following questions about the...Ch. 2 - Prob. 2.101CPCh. 2 - Prob. 2.102CP
Knowledge Booster
Similar questions
- 1. Part 1: Naming Organic Compounds он H₁C-C-CH3 CH3 Br CI CI 2. Br-CH-CH-CH₂ H₂C-CH-C= -CH-CH2-CH3 3. HC-CH-CH-C-OH 5. H₂C-CH-CH₂-OH 7. OH 4. CH CH₂-CH₂ 6. сно CH-CH-CH-CH₂-CH₂ H₁₂C-CH-CH-CH-CH₁₂-CH₁₂ 8. OHarrow_forward11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forwardUnder aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forward
- Assign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forwardPredict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forward
- Predict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward
- 个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forwardPredict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER