
ALEKS 360 ACCESS CARD F/GEN. ORG.CHEM
3rd Edition
ISBN: 9781264452545
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 2.1, Problem 2.5P
Identify the elements used ineach example of molecular art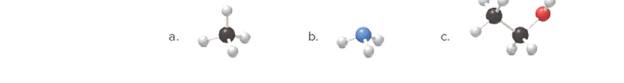
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than
one anomer, you can draw any of them.
Click and drag to start drawing a
structure.
X
Epoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the
mechanism of epoxide opening in aqueous acid.
2nd attempt
Be sure to show all four bonds at stereocenters using hash and wedge lines.
0
0
Draw curved arrows to show how the epoxide reacts with hydronium ion.
100 +1:
1st attempt
Feedback
Be sure to show all four bonds at stereocenters using hash and wedge lines.
See Periodic Table
See Hint
H
A
5
F
F
Hr
See Periodic Table See Hint
03 Question (1 point)
For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry.
>
1. CH₂CH₂MgBr
2. H₂O
3rd attempt
Draw all four bonds at chiral centers. Draw all stereoisomers formed.
Draw the structures here.
e
130
AN
H
See Periodic Table See Hint
P
C
Br
Chapter 2 Solutions
ALEKS 360 ACCESS CARD F/GEN. ORG.CHEM
Ch. 2.1 - Give the symbol for each element. a. calcium, a...Ch. 2.1 - Give the name corresponding to each element...Ch. 2.1 - Locate each element in the periodic table and...Ch. 2.1 - Classify each micronutrient in Figure 2.2 as a...Ch. 2.1 - Identify the elements used ineach example of...Ch. 2.1 - Identify the elements in each chemical formula,...Ch. 2.1 - Prob. 2.7PCh. 2.2 - Prob. 2.8PCh. 2.2 - Prob. 2.9PCh. 2.2 - For the given atom: (a) determine the number of...
Ch. 2.2 - How many protons, neutrons, and electrons are...Ch. 2.2 - What is the mass number of an atom that contains...Ch. 2.3 - For each atom give the following information: [1]...Ch. 2.3 - Magnesium has three isotopes that contain 12, 13,...Ch. 2.3 - Prob. 2.15PCh. 2.3 - Calculate the atomic weight of each element given...Ch. 2.4 - Prob. 2.17PCh. 2.4 - Prob. 2.18PCh. 2.4 - Identify the element fitting each description. an...Ch. 2.4 - Identify each highlighted element in the periodic...Ch. 2.5 - How many electrons are present in each shell,...Ch. 2.6 - What element has each electronic configuration? a....Ch. 2.6 - What element(s) in the first and second period fit...Ch. 2.6 - Draw an orbital diagram for each element; (a)...Ch. 2.6 - Give the electronic configuration for each element...Ch. 2.7 - Prob. 2.26PCh. 2.7 - Determine the number of valence electrons and give...Ch. 2.7 - Prob. 2.28PCh. 2.7 - Give the electron-dot symbol for each element: (a)...Ch. 2.8 - Which element in each pair has the larger atomic...Ch. 2.8 - Prob. 2.31PCh. 2.8 - (a) Which of the indicated atoms has the smaller...Ch. 2 - Identify the elements used in each example of...Ch. 2 - Write a chemical formula for each example of...Ch. 2 - Give the name of the elements in each group of...Ch. 2 - What element(s) are designated by each symbol or...Ch. 2 - Does each chemical formula represent an element or...Ch. 2 - Identify the elements in each chemical formula and...Ch. 2 - Prob. 2.39PCh. 2 - Prob. 2.40PCh. 2 - Give all of the terms that apply to each...Ch. 2 - Give all of the terms that apply to each...Ch. 2 - Give the following information about the atom...Ch. 2 - Give the following information about the atom...Ch. 2 - Prob. 2.45PCh. 2 - Prob. 2.46PCh. 2 - Label each region on the periodic table. Noble...Ch. 2 - Identify each highlighted element in the periodic...Ch. 2 - Prob. 2.49PCh. 2 - Prob. 2.50PCh. 2 - Prob. 2.51PCh. 2 - Prob. 2.52PCh. 2 - Prob. 2.53PCh. 2 - Prob. 2.54PCh. 2 - Prob. 2.55PCh. 2 - Prob. 2.56PCh. 2 - How many protons, neutrons, and electrons are...Ch. 2 - Give the number of protons, neutrons, and...Ch. 2 - Write the element symbol that fits each...Ch. 2 - Write the element symbol that fits each...Ch. 2 - Calculate the atomic weight of silver, which has...Ch. 2 - Calculate the atomic weight of antimony, which has...Ch. 2 - Prob. 2.63PCh. 2 - What is the maximum number of electrons that can...Ch. 2 - Prob. 2.65PCh. 2 - Use an orbital diagram to write the electronic...Ch. 2 - Prob. 2.67PCh. 2 - For each element in Problem 2.66: Write out the...Ch. 2 - Prob. 2.69PCh. 2 - Prob. 2.70PCh. 2 - Give the total number of electrons, the number of...Ch. 2 - Give the total number of electrons, the number of...Ch. 2 - Prob. 2.73PCh. 2 - Prob. 2.74PCh. 2 - Prob. 2.75PCh. 2 - Prob. 2.76PCh. 2 - Prob. 2.77PCh. 2 - Prob. 2.78PCh. 2 - Prob. 2.79PCh. 2 - Prob. 2.80PCh. 2 - Prob. 2.81PCh. 2 - Prob. 2.82PCh. 2 - Prob. 2.83PCh. 2 - Prob. 2.84PCh. 2 - Prob. 2.85PCh. 2 - Prob. 2.86PCh. 2 - Prob. 2.87PCh. 2 - Prob. 2.88PCh. 2 - Rank the atoms in each group in order of...Ch. 2 - Prob. 2.90PCh. 2 - Prob. 2.91PCh. 2 - Prob. 2.92PCh. 2 - Prob. 2.93PCh. 2 - Prob. 2.94PCh. 2 - Prob. 2.95PCh. 2 - (a) What is the chemical formula for...Ch. 2 - Answer the following questions about the...Ch. 2 - Platinum is a precious metal used in a wide...Ch. 2 - Prob. 2.99PCh. 2 - Answer the following questions about the...Ch. 2 - Prob. 2.101CPCh. 2 - Prob. 2.102CP
Additional Science Textbook Solutions
Find more solutions based on key concepts
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Give the IUPAC name for each compound.
Organic Chemistry
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
Physics of Everyday Phenomena
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forwardCalculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forward
- Alcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The reaction mechanism typically involves a carbocation intermediate. > 3rd attempt 3343 10 8 Draw arrows to show the reaction between the alkene and hydronium ion. that 2nd attempt Feedback 1st attempt تعمال Ju See Periodic Table See Hint F D Ju See Periodic Table See Hintarrow_forwardDraw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product. 4th attempt Π Draw the simplified curved arrow mechanism T 3rd attempt Feedback Ju See Periodic Table See Hint H -H H -I H F See Periodic Table See Hintarrow_forwardSelect the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation to show how it is converted to the final product. 4th attempt Part 1 (0.5 point) Select the correct reagent to accomplish the first step of this reaction. Choose one: OA Mg in ethanol (EtOH) OB. 2 Li in THF O C. Li in THF D. Mg in THF O E Mg in H2O Part 2 (0.5 point) Br Part 1 Bri Mg CH B CH, 1 Draw intermediate here, but no arrows. © TE See Periodic Table See Hint See Hint ין Harrow_forward
- Select the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forwardBased on the chart Two similarities between the molecule with alpha glycosidic linkages. Two similarities between the molecules with beta glycosidtic linkages. Two differences between the alpha and beta glycosidic linkages.arrow_forward
- please help fill in the tablearrow_forwardAnswer F pleasearrow_forward4. Refer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A.Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
GCSE Chemistry - Differences Between Compounds, Molecules & Mixtures #3; Author: Cognito;https://www.youtube.com/watch?v=jBDr0mHyc5M;License: Standard YouTube License, CC-BY