
(a)
Interpretation:
The mechanism of 4-Hydroxybenzaldehyde to compound A has to be shown.
(b)
Interpretation:
The reagent and experiment condition is to be given for the conversion of A to B.
Concept introduction:
(c)
Interpretation:
The mechanism for the conversion of B to C is to be explained.
Concept introduction:
Wittig Reaction: It is an organic reaction where an aldehyde or a ketone gets converted to
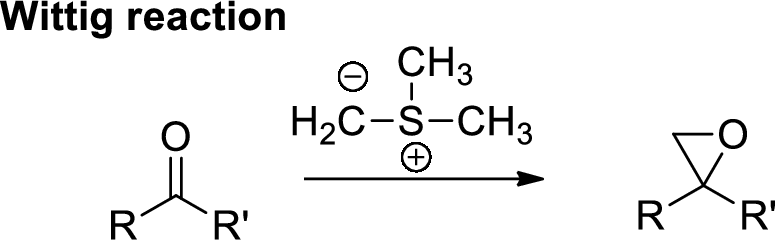
(d)
Interpretation:
The reagent and experiment condition is to be given for the conversion of C to D.
Concept introduction:
(e)
Interpretation:
The reagent and experiment condition is to be given for the conversion of D to albuterol.
Concept introduction:
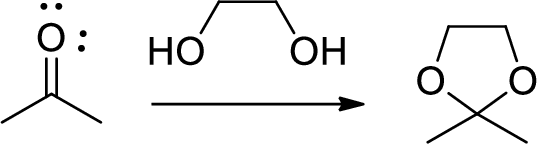
Diol is to protect the ketone and aldehyde (carbonyl group). In this reaction acetone is protected as acetal by using ethylene glycol.
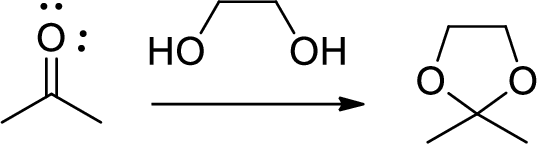
(f)
Interpretation:
The possible stereoisomer’s has to be shown if the product is chiral.
Concept introduction:
Chiral:
A molecule is non superimposable on its mirror image is called chiral molecule. Four different atoms attached to a carbon atom is called chiral molecule.
Isomer: A molecule having the same molecular formula but with different chemical structure is called isomer.
Stereoisomers: Stereoisomers are molecules that have the same molecular formula and they differ only in arrangement of atom in three-dimensional space.
Enantiomers: A compound which is non-superimposable mirror image is called enantiomers.
Diastereomers: A compound which is non-superimposable and non-mirror image is called diastereomers.
Racemic mixture: A racemic mixture is simply a mixture containing an equal amount of each enantiomer.
Trending nowThis is a popular solution!

Chapter 21 Solutions
Organic Chemistry, Loose-leaf Version
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
- Choose the major product of the reaction with correct regio- and stereochemistry. Br2 H₂O O "Br Br & O 'Br OH Br 吡 O OH OH Br "OH Brarrow_forwardSelect the major product of the following reaction. & Br (CH)CONa (CH₂),COH 0 OC(CH) O &arrow_forwardDraw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
