
(a)
Interpretation:
The number of
Concept Introduction:
There are three types of
(a)
Explanation of Solution
The given compound is shown here:
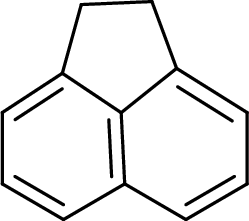
No. of double bonds:
So, the total number of
(b)
Interpretation:
The number of
Concept Introduction:
There are three types of
(b)
Explanation of Solution
The given compound is shown here:
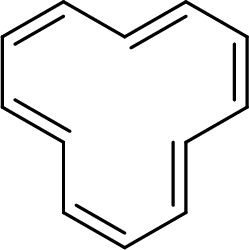
No. of double bonds:
So, the total number of
(c)
Interpretation:
The number of
Concept Introduction:
There are three types of
(c)
Explanation of Solution
The given compound is shown here:
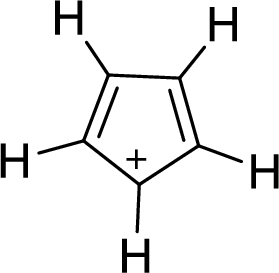
No. of double bonds:
So, the total number of
(d)
Interpretation:
The number of
Concept Introduction:
There are three types of
(d)
Explanation of Solution
The given compound is shown here:
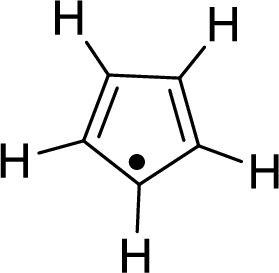
No. of double bonds:
So, the number of
There is an unpaired free-radical electron which is represented by a single dot. This unpaired free-radical electron will be in resonance with the
Therefore, the total number of
(e)
Interpretation:
The number of
Concept Introduction:
There are three types of
(e)
Explanation of Solution
The given compound is shown here:
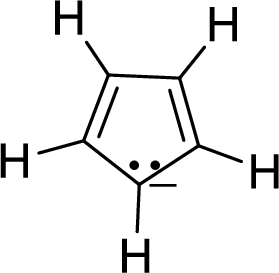
No. of double bonds:
So, the number of
There is a pair of electrons which is represented by two dots with a negative sign. This pair of electrons will be in resonance with the
Therefore, the total number of
(f)
Interpretation:
The number of
Concept Introduction:
There are three types of
(f)
Explanation of Solution
The given compound is shown here:
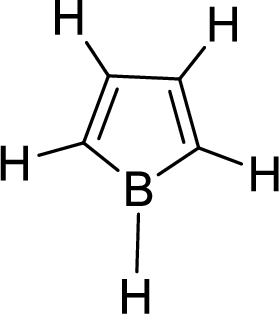
No. of double bonds:
So, the total number of
(g)
Interpretation:
The number of
Concept Introduction:
There are three types of
(g)
Explanation of Solution
The given compound is shown here:
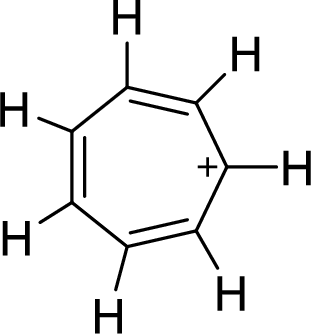
No. of double bonds:
So, the total number of
(h)
Interpretation:
The number of
Concept Introduction:
There are three types of
(h)
Explanation of Solution
The given compound is shown here:
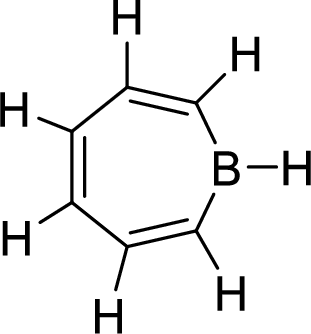
No. of double bonds:
So, the total number of
(i)
Interpretation:
The number of
Concept Introduction:
There are three types of
(i)
Explanation of Solution
The given compound is shown here:
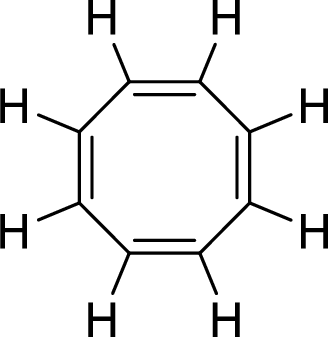
No. of double bonds:
So, the total number of
(j)
Interpretation:
The number of
Concept Introduction:
There are three types of
(j)
Explanation of Solution
The given compound is shown here:
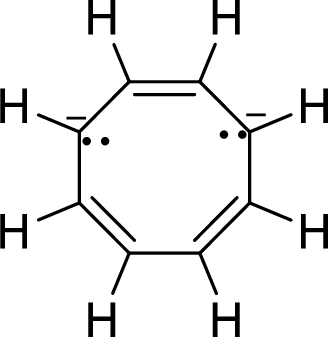
No. of double bonds:
So, the number of
There are two pairs of electrons and each pair is represented by two dots with a negative sign. These two pair of electrons will be in resonance with the
Therefore, the total number of
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry, Loose-leaf Version
- In the table below, the exact chemical structures for Methyl salicylate can be represented by the letter WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR PHRASES CI B) A) E) Cl racemic F) J) CI K) N) OH P) Pool of Reagents for Part B OH OH G) L) OH D) HO H) M) HO Q) R) CIarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardPart I. a) Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone b) Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone (3,3-dimethyl-2-butanone) and 2, 3-dimethyl - 1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forward
- 3. The explosive decomposition of 2 mole of TNT (2,4,6-trinitrotoluene) is shown below: Assume the C(s) is soot-basically atomic carbon (although it isn't actually atomic carbon in real life). 2 CH3 H NO2 NO2 3N2 (g)+7CO (g) + 5H₂O (g) + 7C (s) H a. Use bond dissociation energies to calculate how much AU is for this reaction in kJ/mol.arrow_forwardPart I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forwardShow the mechanism for these reactionsarrow_forward
- Draw the stepwise mechanismarrow_forwardDraw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forward
- Draw stepwise mechanismarrow_forwardPart I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: a) Give the major reason for the exposure of benzophenone al isopropyl alcohol (w/acid) to direct sunlight of pina colone Mechanism For b) Pinacol (2,3-dimethy 1, 1-3-butanediol) on treatment w/ acid gives a mixture (3,3-dimethyl-2-butanone) and 2, 3-dimethyl-1,3-butadiene. Give reasonable the formation of the productsarrow_forwardwhat are the Iupac names for each structurearrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning  Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





