
(a)
Interpretation:
The exergonic portion present in given reaction should be identified.
Concept Introduction:
Exergonic: The reaction is considered as exergonic if energy released since the reactants loses its energy, making the free energy is more negative hence making it spontaneous reaction.
Endergonic: The reaction is considered as endergonic if it needs more energy means that activation energy is much higher making the reaction non spontaneous.
(a)
Answer to Problem 21.20UKC
The exergonic portion is conversion of succinyl phosphate into succinate.
Explanation of Solution
Analyzing the given reaction it is clear that conversion of succinyl phosphate to succinate is exergonic since the phosphate bond in succinyl phosphate gets cleaved which releases energy.
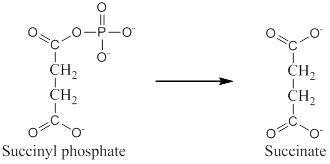
Hence, exergonic portion is conversion of succinyl phosphate into succinate.
(b)
Interpretation:
The endergonic portion present in given reaction should be identified.
Concept Introduction:
Exergonic: The reaction is considered as exergonic if energy released since the reactants loses its energy making the free energy more negative hence making it spontaneous reaction.
Endergonic: The reaction is considered as endergonic if it needs more energy means that activation energy is much higher making the reaction non spontaneous.
(b)
Answer to Problem 21.20UKC
The endergonic portion is conversion of
Explanation of Solution
Analyzing the given reaction it is clear that conversion of
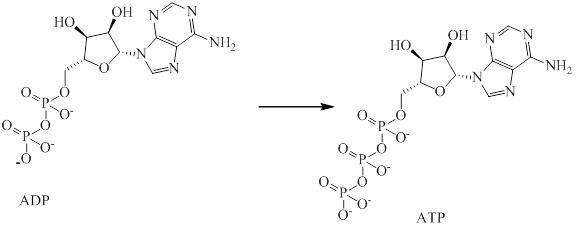
Therefore, addition of phosphate group into
Hence, endergonic portion is conversion of
Want to see more full solutions like this?
Chapter 21 Solutions
Pearson eText Fundamentals of General, Organic, and Biological Chemistry -- Instant Access (Pearson+)
- 2. Which one is the major organic product obtained from the following reaction sequence? HO A OH 1. NaOEt, EtOH 1. LiAlH4 EtO OEt 2. H3O+ 2. H3O+ OH B OH OH C -OH HO -OH OH D E .CO₂Etarrow_forwardwhat is a protein that contains a b-sheet and how does the secondary structure contributes to the overall function of the protein.arrow_forwarddraw and annotate a b-sheet and lable the hydrogen bonding. what is an example that contains the b-sheet and how the secondary structure contributes to the overall function of your example protein.arrow_forward
- Four distinct classes of interactions (inter and intramolecular forces) contribute to a protein's tertiary and quaternary structures. Name the interaction then describe the amino acids that can form this type of interaction. Draw and annotate a diagram of the interaction between two amino acids.arrow_forwardExamine the metabolic pathway. The enzymes that catalyze each step are identified as "e" with a numeric subscript. e₁ e3 e4 A B с 1° B' 02 e5 e6 e7 E F Which enzymes catalyze irreversible reactions? ப e ez ☐ ez e4 ☐ ப es 26 5 e7 Which of the enzymes is likely to be the allosteric enzyme that controls the synthesis of G? €2 ез e4 es 26 5 e7arrow_forwardAn allosteric enzyme that follows the concerted model has an allosteric coefficient (T/R) of 300 in the absence of substrate. Suppose that a mutation reversed the ratio. Select the effects this mutation will have on the relationship between the rate of the reaction (V) and substrate concentration, [S]. ㅁㅁㅁ The enzyme would likely follow Michaelis-Menten kinetics. The plot of V versus [S] would be sigmoidal. The enzyme would mostly be in the T form. The plot of V versus [S] would be hyperbolic. The enzyme would be more active.arrow_forward
- Penicillin is hydrolyzed and thereby rendered inactive by penicillinase (also known as ẞ-lactamase), an enzyme present in some penicillin-resistant bacteria. The mass of this enzyme in Staphylococcus aureus is 29.6 kDa. The amount of penicillin hydrolyzed in 1 minute in a 10.0 mL. solution containing 1.00 x 10 g of purified penicillinase was measured as a function of the concentration of penicillin. Assume that the concentration of penicillin does not change appreciably during the assay. Plots of V versus [S] and 1/V versus 1/[S] for these data are shown. Vo (* 10 M minute"¹) 7.0 6.0 5.0 4.0 3.0 20 1.0 0.0 о 10 20 30 1/Vo (* 10 M1 minute) 20 103 90 BO 70 50 [S] (* 100 M) 40 50 60 y=762x+1.46 × 10" [Penicillin] (M) Amount hydrolyzed (uM) 1 0.11 3 0.25 5 0.34 10 0.45 30 0.58 50 0.61arrow_forwardConsider the four graphs shown. In each graph, the solid blue curve represents the unmodified allosteric enzyme and the dashed green curve represents the enzyme in the presence of the effector. Identify which graphs correctly illustrate the effect of a negative modifier (allosteric inhibitor) and a positive modifier (allosteric activator) on the velocity curve of an allosteric enzyme. Place the correct graph in the set of axes for each type of modifier. Negative modifier Reaction velocity - Positive modifier Substrate concentration - Reaction velocity →→→→ Substrate concentration Answer Bankarrow_forwardConsider the reaction: phosphoglucoisomerase Glucose 6-phosphate: glucose 1-phosphate After reactant and product were mixed and allowed to reach at 25 °C, the concentration of each compound at equilibrium was measured: [Glucose 1-phosphate] = 0.01 M [Glucose 6-phosphate] = 0.19 M Calculate Keq and AG°'. Код .0526 Incorrect Answer 7.30 AG°' kJ mol-1 Incorrect Answerarrow_forward
- Classify each phrase as describing kinases, phosphatases, neither, or both. Kinases Phosphatases Neither Both Answer Bank transfer phosphoryl groups to acidic amino acids in eukaryotes may use ATP as a phosphoryl group donor remove phosphoryl groups from proteins catalyze reactions that are the reverse of dephosphorylation reactions regulate the activity of other proteins catalyze phosphorylation reactions PKA as an example turn off signaling pathways triggered by kinasesarrow_forwardConsider the reaction. kp S P kg What effects are produced by an enzyme on the general reaction? AG for the reaction increases. The rate constant for the reverse reaction (kr) increases. The reaction equilibrium is shifted toward the products. The concentration of the reactants is increased. The activation energy for the reaction is lowered. The formation of the transition state is promoted.arrow_forwardThe graph displays the activities of wild-type and several mutated forms of subtilisin on a logarithmic scale. The mutations are identified as: • The first letter is the one-letter abbreviation for the amino acid being altered. • The number identifies the position of the residue in the primary structure. ⚫ The second letter is the one-letter abbreviation for the amino acid replacing the original one. • Uncat. refers to the estimated rate for the uncatalyzed reaction. Log₁(S-1) Wild type S221A H64A -5 D32A S221A H64A D32A -10 Uncat. How would the activity of a reaction catalyzed by a version of subtilisin with all three residues in the catalytic triad mutated compare to the activity of the uncatalyzed reaction? It would have more activity, because the reaction catalyzed by the triple mutant is approximately three-fold faster than the uncatalyzed reaction. It would have less activity, because the reaction catalyzed by the triple mutant is approximately 1000-fold slower than the…arrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax





