
Chemistry Atoms First2e
2nd Edition
ISBN: 9781947172647
Author: OpenStax
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 12E
Give the complete IUPAC name for each of the following compounds:
(a) (CH3)2CHF.
(b) CH3CHClCHClCH3
(C)
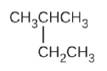
(d) CH3CH2CH = CHCH3
(e)
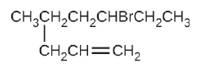
(f)
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
help me solve this HW
Molecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)
Indicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.
Chapter 21 Solutions
Chemistry Atoms First2e
Ch. 21 - Write the chemical formula and Lewis structure of...Ch. 21 - What is the difference between the hybridization...Ch. 21 - On a microscopic level, how does the reaction of...Ch. 21 - On a microscopic level, how does the reaction of...Ch. 21 - Explain why unbranched alkenes can form geometric...Ch. 21 - Explain why these two molecules are not isomers:Ch. 21 - Explain why these two molecules are not isomers:Ch. 21 - How does the carbon-atom hybridization change when...Ch. 21 - Write the Lewis structure and molecular formula...Ch. 21 - Write the chemical formula, condensed formula, and...
Ch. 21 - Give the complete IUPAC name for each of the...Ch. 21 - Give the complete IUPAC name for each of the...Ch. 21 - Butane is used as a fuel in disposable lighters....Ch. 21 - Write Lewis structures and name the five...Ch. 21 - Write Lewis structures for the Cis -trans isomers...Ch. 21 - Write structures for the three isomers of the...Ch. 21 - Isooctane is the common name of the isomer of...Ch. 21 - Write Lewis structures and IUPAC names for the...Ch. 21 - Write Lewis structures and IUPAC names for all...Ch. 21 - Name and write the structures of all isomers of...Ch. 21 - Write the structures for all the isomers of the...Ch. 21 - Write Lewis structures and describe the molecular...Ch. 21 - Benzene is one of the compounds used as an octane...Ch. 21 - Teflon is prepared by the polymerization of...Ch. 21 - Write two complete, balanced equations for each of...Ch. 21 - Write two complete, balanced equations for each of...Ch. 21 - What mass of 2-bromopropane could be prepared from...Ch. 21 - Acetylene is a very weak acid; however, it will...Ch. 21 - Ethylene can be produced by the pyrolysis of...Ch. 21 - Why do the compounds hexane, hexanol, and hexane...Ch. 21 - Write condensed formulas and provide IUPAC names...Ch. 21 - Give the complete IUPAC name for each of the...Ch. 21 - Give the complete IUPAC name and the common name...Ch. 21 - Write the condensed structures of both isomers...Ch. 21 - Write the condensed structures of all isomers with...Ch. 21 - Draw the condensed formulas for each of the...Ch. 21 - MTBE, Methyl tert -butyl ether, CH3OC(CH3)3, is...Ch. 21 - Write two complete balanced equations for each of...Ch. 21 - Write two complete balanced equations for each of...Ch. 21 - Order the following molecules from least to most...Ch. 21 - Predict the products of oxidizing the molecules...Ch. 21 - Predict the products of reducing the following...Ch. 21 - Explain why it is not possible to possible a...Ch. 21 - How does hybridization of the substituted carbon...Ch. 21 - Fatty acids are carboxylic acids that have long...Ch. 21 - Write a condensed structural formula, such as...Ch. 21 - Write a condensed structural formula, such as...Ch. 21 - The foul odor of rancid butter is caused by...Ch. 21 - Write the two-resonance structures for the acetate...Ch. 21 - Write two complete, balanced equations for each of...Ch. 21 - Write two complete balanced equations for each of...Ch. 21 - Yields in organic reactions are sometimes low....Ch. 21 - Alcohols A, B and C all have the composition C4H...Ch. 21 - Write the Lewis structures of both isomers with...Ch. 21 - What is the molecular structure about the nitrogen...Ch. 21 - Write the two resonance structures for the...Ch. 21 - Draw Lewis structures for pyridine and its...Ch. 21 - Write the Lewis structures of all isomers with the...Ch. 21 - Write two complete balanced equations for the...Ch. 21 - Write two complete, balanced equations for each of...Ch. 21 - Identify any carbon atoms that change...Ch. 21 - Identify any carbon atoms that change...Ch. 21 - Identify any carbon atoms that change...
Additional Science Textbook Solutions
Find more solutions based on key concepts
In rats, the following genotypes of two independently assorting autosomal genes determine coat color: A third g...
Concepts of Genetics (12th Edition)
Look at the relative positions of each pair of atoms listed here in the periodic table. How many core electrons...
Organic Chemistry (8th Edition)
Compare and contrast aerobic respiration, anaerobic respiration, and fermentation.
Microbiology with Diseases by Body System (5th Edition)
Some organizations are starting to envision a sustainable societyone in which each generation inherits sufficie...
Campbell Essential Biology (7th Edition)
85. Choose the more metallic element from each pair.
a. Sr or Sb
b. As or Bi
c. Cl or O
d. S or As
Introductory Chemistry (6th Edition)
In the following diagram, the white spheres represent hydrogen atoms and the blue Sphere represent the nitrogen...
Chemistry: The Central Science (14th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forwardWhat characteristics should an interface that forms an electrode have?arrow_forward
- For a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forwardDescribe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forward
- State two similarities between fluorescence and phosphorescence.arrow_forwardState three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forwardIn a photochemical reaction, how is the rate of the process related to its quantum yield?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY