
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
9th Edition
ISBN: 9781292151229
Author: Wade, LeRoy G.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20.2C, Problem 20.2P
Name the following
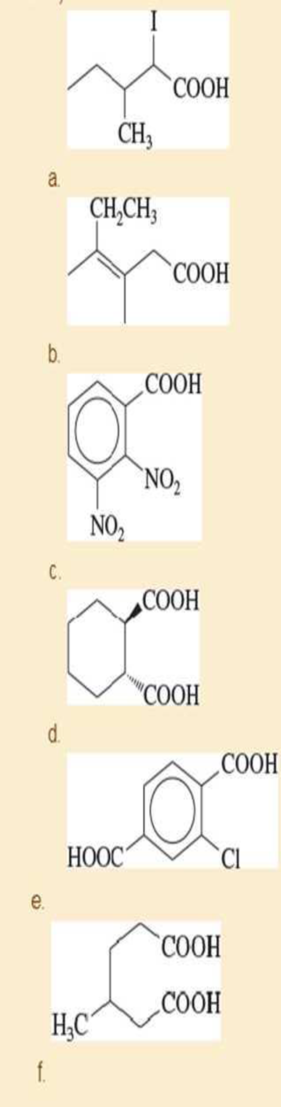
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Correctly name this compound using the IUPAC naming system by sorting the
components into the correct order.
Br
IN
Ν
H
How is the radical intermediate for this structure formed? Can you please draw arrows from the first radical to the resonance form that would result in this product? I'm lost.
Part VI.
(a) calculate the λ max of the compound using woodward - Fieser rules.
(b) what types of electronic transitions are present in the compound?
(c) what are the prominent peaks in the IR spectrum of the compound?
Chapter 20 Solutions
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
Ch. 20.2C - Prob. 20.1PCh. 20.2C - Name the following carboxylic acids (when...Ch. 20.4B - Rank the compounds in each set in order of...Ch. 20.5 - Prob. 20.4PCh. 20.5 - Phenols are less acidic than carboxylic acids,...Ch. 20.5 - Prob. 20.6PCh. 20.7A - Prob. 20.7PCh. 20.7B - Prob. 20.8PCh. 20.7D - Draw all four resonance forms of the fragment at...Ch. 20.7D - a. Why do most long-chain fatty acids show a large...
Ch. 20.10 - Prob. 20.13PCh. 20.10 - A carboxylic acid has two oxygen atoms, each with...Ch. 20.10 - Prob. 20.15PCh. 20.10 - The mechanism of the Fischer esterification was...Ch. 20.10 - Prob. 20.17PCh. 20.12 - Show how to synthesize the following compounds,...Ch. 20.13 - Show how you would synthesize the following...Ch. 20.14 - Prob. 20.20PCh. 20.14 - Prob. 20.21PCh. 20.15 - Propose a mechanism for the reaction of benzoic...Ch. 20.15 - Prob. 20.23PCh. 20.15 - Prob. 20.24PCh. 20 - Prob. 20.25SPCh. 20 - Give both IUPAC names and common names for the...Ch. 20 - Draw the structures of the following compounds. a....Ch. 20 - Prob. 20.28SPCh. 20 - Arrange each group of compounds in order of...Ch. 20 - Predict the products (if any) of the following...Ch. 20 - Rank the following isomers in order of increasing...Ch. 20 - Prob. 20.32SPCh. 20 - What do the following pKa values tell you about...Ch. 20 - Given the structure of ascorbic acid (vitamin C):...Ch. 20 - Prob. 20.35SPCh. 20 - Show how you would accomplish the following...Ch. 20 - Predict the products and propose mechanisms for...Ch. 20 - Prob. 20.38SPCh. 20 - Prob. 20.39SPCh. 20 - Prob. 20.40SPCh. 20 - Prob. 20.44SPCh. 20 - Prob. 20.45SPCh. 20 - Predict the major form of each compound when it is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward↑ 0 Quiz List - RCC430M_RU05 X Aktiv Learning App × Qdraw resonance structure ×Q draw resonance structure xb My Questions | bartleby ×+ https://app.aktiv.com Draw a resonance structure of pyrrole that has the same number of pi bonds as the original structure. Include all lone pairs in your structure. + N H a 5 19°F Cloudy Q Search Problem 12 of 15 Atoms, Bonds and Rings Charges and Lone Pairs myhp हजु Undo Reset Remove Done Submit Drag To Pan 2:15 PM 1/25/2025arrow_forward
- Briefly indicate the structure and bonding of silicates.arrow_forward4 Part C Give the IUPAC name and a common name for the following ether: Spell out the full names of the compound in the indicated order separated by a comma.arrow_forwardTry: Draw possible resonance contributing structures for the following organic species: CH3CH2NO2 [CH2CHCH2] [CH2CHCHO] [CH2CHCH2] [CH2CHNH2]arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY