
Concept explainers
Interpretation:
The question asks one to write the equations corresponding to the oxidation of a primary alcohol and a secondary alcohol. Also one needs to specify the structure of each alcohol and the principal products of oxidation.
Concept Introduction:
Monohydric alcohols can be categorized into three types as primary, secondary and tertiary alcohols.
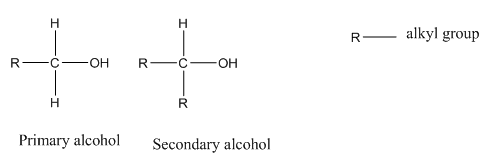
AS shown above in primary alcohols carbon with the −OH group is bonded to only one carbon whereas in secondary alcohols, carbon with the −OH group is bonded to two other carbon atoms. In tertiary alcohols(not shown here), carbon with the −OH group is bonded to three other carbon atoms.
In the alcohols the −OH bond is polarized placing partially negative charge on O atom while placing a partially positive charge on the alkyl carbon atom of the R-OH bond. Hence due to the inter molecular hydrogen bonds formed between alcohol molecules, their boiling points have higher values compared to the
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
EBK INTRODUCTORY CHEMISTRY
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





