
Pearson eText Fundamentals of General, Organic, and Biological Chemistry -- Instant Access (Pearson+)
8th Edition
ISBN: 9780135213759
Author: John McMurry, David Ballantine
Publisher: PEARSON+
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 20.49AP
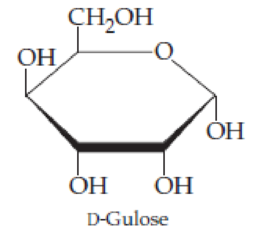
D-Gulose, an aldohexose isomer of glucose, has the cyclic structure shown here. Which is shown, the α form or the β form?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Assessment
+1501 pts
/1600
Resources
Solution
? Hint
Sub
bo Each pictured Lewis structure is invalid. Identify the error in each case.
O Macmillan Learning
:0▬▬0:
Answer Bank
wrong electron total
:0-
:F======F:
octet-rule violation
N
:0:
[s] mM
V (M/s)
Uninhibited
0.333
1.65 x 107
1.05 x 107
V (M/s) x 10'
Inhibitor A
V (M/s) x 107
Inhibitor B
0.794 x 107
0.40
1.86 x 107
1.21 x 107
0.893 x 107
0.50
2.13 x 107
1.43 x 107
1.02 x 107
0.666
2.49 x 107
1.74 x 107
1.19 x 107
1.0
2.99 x 107
2.22 x 107
1.43 x 107
2.0
3.72 x 107
3.08 x 107
1.79 x 107
For a Michaelis-Menten reaction, k₁-5 x 10'/M-s, k.-2 x 10%/s, and k₂-4 x 10²/s.
a)
Calculate the Ks and KM for this reaction.
b)
Does substrate binding achieve equilibrium or steady state?
Chapter 20 Solutions
Pearson eText Fundamentals of General, Organic, and Biological Chemistry -- Instant Access (Pearson+)
Ch. 20.1 - Classify the following monosaccharides as an...Ch. 20.1 - Prob. 20.2PCh. 20.2 - Prob. 20.3PCh. 20.2 - Prob. 20.4PCh. 20.2 - Prob. 20.6PCh. 20.3 - D-Talose, a constituent of certain antibiotics,...Ch. 20.3 - Prob. 20.8PCh. 20.3 - Draw the structure that completes the mutarotation...Ch. 20.4 - Prob. 20.10KCPCh. 20.4 - Prob. 20.11P
Ch. 20.4 - Prob. 20.12PCh. 20.4 - Prob. 20.13PCh. 20.4 - Prob. 20.1CIAPCh. 20.4 - Prob. 20.2CIAPCh. 20.4 - All cells in your body contain glycoproteins...Ch. 20.5 - Draw the structure of the and anomers that...Ch. 20.6 - Prob. 20.15PCh. 20.6 - Prob. 20.16PCh. 20.6 - Prob. 20.17KCPCh. 20.7 - Prob. 20.4CIAPCh. 20.7 - Prob. 20.5CIAPCh. 20.7 - Prob. 20.6CIAPCh. 20.7 - Prob. 20.7CIAPCh. 20.7 - Prob. 20.18PCh. 20.7 - Prob. 20.19PCh. 20.7 - Prob. 20.8CIAPCh. 20.7 - Prob. 20.9CIAPCh. 20.7 - Prob. 20.10CIAPCh. 20 - During the digestion of starch from potatoes, the...Ch. 20 - Prob. 20.21UKCCh. 20 - Consider the trisaccharide A, B, C shown in...Ch. 20 - Hydrolysis of both glycosidic bonds in the...Ch. 20 - Prob. 20.24UKCCh. 20 - Are one or more of the disaccharides maltose,...Ch. 20 - Prob. 20.26UKCCh. 20 - Prob. 20.27UKCCh. 20 - Prob. 20.28APCh. 20 - What is the family-name ending for a sugar?Ch. 20 - Prob. 20.30APCh. 20 - Classify the four carbohydrates (a)(d) by...Ch. 20 - Prob. 20.32APCh. 20 - How many chiral carbon atoms are there in each of...Ch. 20 - Prob. 20.34APCh. 20 - Prob. 20.35APCh. 20 - Name four important monosaccharides and tell where...Ch. 20 - Prob. 20.37APCh. 20 - Prob. 20.38APCh. 20 - What is the structural relationship between...Ch. 20 - Prob. 20.40APCh. 20 - In Section 15.6, you saw that aldehydes react with...Ch. 20 - Sucrose and D-glucose rotate plane-polarized light...Ch. 20 - Prob. 20.43APCh. 20 - Prob. 20.44APCh. 20 - Prob. 20.45APCh. 20 - What is mutarotation? Do all chiral molecules do...Ch. 20 - What are anomers, and how do the anomers of a...Ch. 20 - What is the structural difference between the ...Ch. 20 - D-Gulose, an aldohexose isomer of glucose, has the...Ch. 20 - Prob. 20.50APCh. 20 - In its open-chain form, D-altrose has the...Ch. 20 - Prob. 20.52APCh. 20 - Prob. 20.53APCh. 20 - Prob. 20.54APCh. 20 - Prob. 20.55APCh. 20 - What is the structural difference between a...Ch. 20 - What are glycosides, and how can they be formed?Ch. 20 - Prob. 20.58APCh. 20 - Prob. 20.59APCh. 20 - Give the names of three important disaccharides....Ch. 20 - Lactose and maltose are reducing disaccharides,...Ch. 20 - Amylose (a form of starch) and cellulose are both...Ch. 20 - Prob. 20.63APCh. 20 - Prob. 20.64APCh. 20 - Prob. 20.65APCh. 20 - Gentiobiose, a rare disaccharide found in saffron,...Ch. 20 - Prob. 20.67APCh. 20 - Prob. 20.68APCh. 20 - Prob. 20.69APCh. 20 - Amylopectin (a form of starch) and glycogen are...Ch. 20 - What is the physiological purpose of starch in a...Ch. 20 - Prob. 20.72APCh. 20 - Prob. 20.73APCh. 20 - Prob. 20.74CPCh. 20 - Prob. 20.75CPCh. 20 - Prob. 20.76CPCh. 20 - Prob. 20.77CPCh. 20 - Prob. 20.78CPCh. 20 - Write the open-chain structure of the only...Ch. 20 - Prob. 20.80CPCh. 20 - Prob. 20.81CPCh. 20 - When a person cannot digest galactose, its reduced...Ch. 20 - Describe the differences between mono-, di-, and...Ch. 20 - Prob. 20.84CPCh. 20 - Prob. 20.85CPCh. 20 - Many people who are lactose intolerant can eat...Ch. 20 - Prob. 20.87GPCh. 20 - Prob. 20.88GPCh. 20 - Prob. 20.89GP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Assume that an enzyme-catalyzed reaction follows the scheme shown: E+S SES →E + P k₁ = 1 x 109/M-s k-1=2.5 x 10%/s k₂ = 3.4 x 107/s What is the dissociation constant for the enzyme-substrate, K,? What is the Michaelis constant, Km, for this enzyme? What is the turnover number, Keat, for this enzyme? What is the catalytic efficiency for the enzyme? If the initial Et concentration is 0.25mM, what is Vmax?arrow_forwardAn enzyme lowers the activation energy, (AG) of a reaction from 50.0 kcal/mol to 40.0 kcal/mol. Calulate the catalytic power at 310K. (R-1.987x10 kcal/mol)arrow_forwardDraw a typical axodendritic synapse, including a specific neurotransmitter of your choice, its associated postsynaptic receptors (indicating whether they are ionotropic or metabotropic), and any associated reuptake transporters or degradation enzymes. Please include a description of what specific steps would occur as an action potential reaches the axonal terminal.arrow_forward
- Give a full arrow pushing mechanism of the spontaneous redox reaction between NAD+/NADH and oxaloacetate/malate. Please include diagram drawing of the mechanism! (Thank You!)arrow_forward18. Which one of the compounds below is the major organic product obtained from the following series of reactions? 1. BH3 2. H2O2, NaOH H₂CrO4 CH2N2 oro ororos A B C D Earrow_forward17. Which one of the compounds below is the major organic product obtained from the following series of reactions? CI benzyl alcohol OH PBr3 Mg 1. CO2 SOCl2 ? ether 2. H+, H₂O CI Cl HO OH CI Cl A B C D Earrow_forward
- 14. What is the IUPAC name of this compound? A) 6-hydroxy-4-oxohexanenitrile B) 5-cyano-3-oxo-1-pentanol C) 5-cyano-1-hydroxy-3-pentanone D) 1-cyano-5-hydroxy-3-pentanone E) 5-hydroxy-3-oxopentanenitrile HO. CNarrow_forward13. What is the IUPAC name of this compound? A) 5-hydroxy-3,3-dimethylpentanoic acid B) 3,3-dimethylpentanoic acid C) 3,3-dimethyl-1-oxo-1,5-pentanediol D) 1,5-dihydroxy-3,3-dimethylpentanal E) 4-hydroxy-2,2-dimethylbutanoic acid HO OHarrow_forwardHelp me understand how carbon disulfide leads to toxicity in the brain, using terms like distal axonopathy, neurofilaments, covalent cross-linking, adducts, etc.,...please intuitively explain what is happening and where and the effects of it. For example, I know that CS2 reacts with amide and sulfhydryl groups on proteins, but what proteins exactly and where are they located?arrow_forward
- What is the standard free energy change (in kJ/mole) of the spontaneous reaction between Oxygen and NADH to form H2O2 and NAD+?arrow_forwardRedox Chemistry: Give standard free energy changes expected for the following reactions:-Succinate -> fumarate (using FAD/FADH2)-Oxaloacetate -> Malate (using NAD/NADH)-NADH --> NAD+ (using FMN/FMNH2)-CoQ --> CoQH2 (using Cytochrome C)arrow_forwardGive examples of balanced redox reactions that match the following:-Catabolic-Anabolic-Oxidative-Reductivearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning

Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning

Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College


Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning
Metabolic Pathways; Author: Wisc-Online;https://www.youtube.com/watch?v=m61bQYio9ys;License: Standard Youtube License