
University Physics with Modern Physics (14th Edition)
14th Edition
ISBN: 9780321973610
Author: Hugh D. Young, Roger A. Freedman
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 20, Problem 20.46P
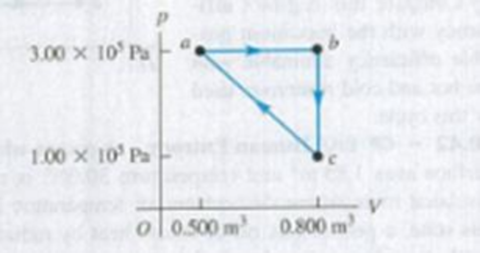
A monatomic ideal gas it taken around the cycle shown in Fig P20.46 in the direction shown in the figure. The path for process c → a is a straight line in the pV-diagram. (a) Calculate Q, W, and ΔU for each process a → b, b → c, and c → a. (b) What are Q, W, and ΔU for one complete cycle? (c) What is the efficiency of the cycle?
Figure P20.46
Expert Solution & Answer
Learn your wayIncludes step-by-step video

schedule11:05
Chapter 20 Solutions
University Physics with Modern Physics (14th Edition)
Ch. 20.1 - Your left and right hands are normally at the same...Ch. 20.2 - Rank the following heat engines in order from...Ch. 20.3 - For an Otto-cycle engine with cylinders of a fixed...Ch. 20.4 - Can you cool your house by leaving the...Ch. 20.5 - Would a 100%-efficient engine (Fig. 20.11a)...Ch. 20.6 - An inventor looking for financial support comes to...Ch. 20.7 - Suppose 2.00 kg of water at 50C spontaneously...Ch. 20.8 - A quantity of N molecules of an ideal gas...Ch. 20 - A pot is half-filled with water, and a lid is...Ch. 20 - Prob. 20.2DQ
Ch. 20 - Prob. 20.3DQCh. 20 - Prob. 20.4DQCh. 20 - Why must a room air conditioner be placed in a...Ch. 20 - Prob. 20.6DQCh. 20 - Prob. 20.7DQCh. 20 - An electric motor has its shaft coupled to that of...Ch. 20 - When a wet cloth is hung up in a hot wind in the...Ch. 20 - Compare the pV-diagram for the Otto cycle in Fig....Ch. 20 - The efficiency of heat engines is high when the...Ch. 20 - What would be the efficiency of a Carnot engine...Ch. 20 - Real heat engines, like the gasoline engine in a...Ch. 20 - Does a refrigerator full of food consume more...Ch. 20 - In Example 20.4, a Carnot refrigerator requires a...Ch. 20 - How can the thermal conduction of heat from a hot...Ch. 20 - Explain why each of the following processes is an...Ch. 20 - The free expansion of an ideal gas is an adiabatic...Ch. 20 - Are the earth and sun in thermal equilibrium? Are...Ch. 20 - Prob. 20.20DQCh. 20 - Prob. 20.21DQCh. 20 - Prob. 20.22DQCh. 20 - BIO A growing plant creates a highly complex and...Ch. 20 - A diesel engine performs 2200 J of mechanical work...Ch. 20 - An aircraft engine takes in 9000 J of heat and...Ch. 20 - A Gasoline Engine. A gasoline engine takes in 1.61...Ch. 20 - A gasoline engine has a power output of 180 kW...Ch. 20 - The pV-diagram in Fig. E20.5 shows a cycle of heat...Ch. 20 - (a) Calculate the theoretical efficiency for an...Ch. 20 - The Otto-cycle engine in a Mercedes-Benz SL1 a...Ch. 20 - Section 20.4 Refrigerators 20.8The coefficient of...Ch. 20 - A refrigerator has a coefficient of performance of...Ch. 20 - A freezer has a coefficient of performance of...Ch. 20 - A refrigerator has a coefficient of performance of...Ch. 20 - A Carnot engine is operated between two heat...Ch. 20 - A Carnot engine whose high-temperature reservoir...Ch. 20 - An ice-making machine operates in a Carnot cycle....Ch. 20 - A Carnot engine has an efficiency of 66% and...Ch. 20 - A certain brand of freezer is advertised to use...Ch. 20 - A Carnot refrigerator is operated between two heat...Ch. 20 - A Carnot heat engine uses a hot reservoir...Ch. 20 - You design an engine that takes in 1.50 104 J of...Ch. 20 - A 4.50-kg block of ice at 0.00C falls into the...Ch. 20 - A sophomore with nothing better to do adds heat to...Ch. 20 - CALC You decide to take a nice hot bath but...Ch. 20 - A 15.0-kg block of ice at 0.0C melts to liquid...Ch. 20 - CALC You make tea with 0.250 kg of 85.0C water and...Ch. 20 - Three moles of an ideal gas undergo a reversible...Ch. 20 - What is the change in entropy of 0.130 kg of...Ch. 20 - (a) Calculate the change in entropy when 1.00 kg...Ch. 20 - Entropy Change Due to Driving. Premium gasoline...Ch. 20 - CALC Two moles of an ideal gas occupy a volume V....Ch. 20 - A box is separated by a partition into two parts...Ch. 20 - CALC A lonely party balloon with a volume of 2.40...Ch. 20 - You are designing a Carnot engine that has 2 mol...Ch. 20 - CP An ideal Carnot engine operates between 500C...Ch. 20 - Prob. 20.34PCh. 20 - CP A certain heat engine operating on a Carnot...Ch. 20 - A heat engine takes 0.350 mol of a diatomic ideal...Ch. 20 - Prob. 20.37PCh. 20 - What is the thermal efficiency of an engine that...Ch. 20 - CALC You build a heal engine that takes 1.00 mol...Ch. 20 - CP As a budding mechanical engineer, you are...Ch. 20 - CALC A heal engine Operates using the cycle shown...Ch. 20 - CP BIO Humun Entropy. A person who has skin of...Ch. 20 - An experimental power plant at the Natural Energy...Ch. 20 - CP BIO A Human Engine. You decide to use your body...Ch. 20 - CALC A cylinder contains oxygen at a pressure of...Ch. 20 - A monatomic ideal gas it taken around the cycle...Ch. 20 - A Carnot engine operates between two heat...Ch. 20 - A typical coal-fired power plant generates 1000 MW...Ch. 20 - Automotive Thermodynamics. A Volkswagen Passat has...Ch. 20 - An air conditioner operates on 800 W of power and...Ch. 20 - The pV-diagram in Fig. P20.51 shows the cycle for...Ch. 20 - BIO Human Entropy. A person with skin of surface...Ch. 20 - CALC An object of mass m1, specific heat c1, and...Ch. 20 - CALC To heat 1 cup of water (250 cm3) to make...Ch. 20 - DATA In your summer job with a venture capital...Ch. 20 - DATA For a refrigerator or air conditioner, the...Ch. 20 - DATA You are conducting experiments to study...Ch. 20 - Consider a Diesel cycle that starts (at point a in...Ch. 20 - POWER FROM THE SEA. Ocean thermal energy...Ch. 20 - POWER FROM THE SEA. Ocean thermal energy...Ch. 20 - POWER FROM THE SEA. Ocean thermal energy...Ch. 20 - POWER FROM THE SEA. Ocean thermal energy...
Additional Science Textbook Solutions
Find more solutions based on key concepts
In Fig. 20.29, take q1 = 68 C, q2 = 34 C, and q3 = 15 C. Find the electric force on q3. FIGURE 20.29 Problems ...
Essential University Physics: Volume 2 (3rd Edition)
The pV-diagram of the Carnot cycle.
Sears And Zemansky's University Physics With Modern Physics
A thin plate has a round hole whose diameter in its rest frame is D. The plate is parallel to the ground and mo...
Modern Physics
Your medieval history class is constructing a trebuchet, a catapult-like weapon for hurling stones at enemy cas...
Essential University Physics: Volume 1 (3rd Edition)
Write each number in scientific notation.
13. 540,000
Applied Physics (11th Edition)
15.60 A continuous succession of sinusoidal wave pulses are produced at one end of a very long string and trave...
University Physics (14th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- (a) What is the hot reservoir temperature of a Carnot engine that has an eficiency of 42.0% and a cold reservoir temperature of 210C ? (b) What must the hot reservoir temperature be for a real heat engine that achieves 0.700 of the maximum eficiency, but still has an efficiency of 42.0% (and a cold reservoir at 27.0C )? (c) Does your answer imply practical limits to the efficiency of car gasoline engines?arrow_forwardA thermal engine produces 4 MJ of electrical energy while operating between two thermal baths of different temperatures. The working substance of the engine discharges 5 MJ of heat to the cold temperature bath. What is the efficiency of the engine?arrow_forwardFigure P22.73 illustrates the cycle ABCA for a 2.00-mol sample of an ideal diatomic gas, where the process CA is a reversible isothermal expansion. What is a. the net work done by the gas during one cycle? b. How much energy is added to the gas by heat during one cycle? c. How much energy is exhausted from the gas by heat during one cycle? d. What is the efficiency of the cycle? e. What would be the efficiency of a Carnot engine operated between the temperatures at points A and B during each cycle?arrow_forward
- A sample of a monatomic ideal gas is contained in a cylinder with a piston. Its stale is represented by the dot in the PV diagram shown in Figure OQ22.9. Arrows A through E represent isobaric, isothermal, adiabatic, and isovolumetric processes that the sample can undergo. In each process except D, the volume changes by a factor of 2. All five processes are reversible. Rank the processes according to the change in entropy of the gas from the largest positive value to the largest-magnitude negative value. In your rankings, display any cases of equality.arrow_forward(a) On a winter day, a certain house loses 5.00108J of heat to the outside (about 500,000 Btu). What is the total change in entropy due to this heat transfer alone, assuming an average indoor temperature of 21.0C and an average outdoor temperature of 5.00C ? (b) This large change in entropy implies a large amount of energy has become unavailable to do work. Where do we find more energy when such energy is lost to us?arrow_forwardAssume a sample of an ideal gas is at room temperature. What action will necessarily make the entropy of the sample increase? (a) Transfer energy into it by heat. (b) Transfer energy into it irreversibly by heat. (c) Do work on it. (d) Increase either its temperature or its volume, without letting the other variable decrease. (e) None of those choices is correct.arrow_forward
- A sample of a monatomic ideal gas is contained in a cylinder with a piston. Its state is represented by the dot in the PV diagram shown in Figure OQ18.9. Arrows A through E represent isobaric, isothermal, adiabatic, and isovolumetric processes that the sample can undergo. In each process except D, the volume changes by a factor of 2. All five processes are reversible. Rank the processes according to the change in entropy of the gas from the largest positive value to the largest-magnitude negative value. In your rankings, display any cases of equality. Figure OQ18.9arrow_forward(a) What is the best coefficient of performance for a refrigerator that cools an environment at 30.0C and has heat transfer to another environment at 45.0C ? (b) How much work in joules must be done for a heat transfer of 4186 kJ from the cold environment? (c) What is the cost of doing this if the work costs 10.0 cents per 3.60106J (a kilowatthour)? (d) How many kJ of heat transfer occurs into the warm environment? (e) Discuss what type of refrigerator might operate between these temperatures.arrow_forwardUse a PV diagram such as the one in Figure 22.2 (page 653) to figure out how you could modify an engine to increase the work done.arrow_forward
- A copper rod of cross-sectional area 5.0 cm2 and length 5.0 m conducts heat from a heat reservoir at 373 K to one at 273 K. What is the time rate of change of the universe's entropy for this process?arrow_forwardA thermodynamic cycle is shown in Figure P21.34 for a gas in a piston. The system changes states along the path ABCA. a. What is the total work done by the gas during this cycle? b. How much heat is transferred? Does heat flow into or out of the system? Figure P21.34arrow_forwardA monoatomic ideal gas (n moles) goes through a cyclic process shown below. Find the change in entropy of the gas in each step and the total entropy change over the entire cycle.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY