
POWER FROM THE SEA.
Ocean thermal energy conversion is a process that uses the temperature difference between the warm surface water of tropical oceans and the cold deep-ocean water to run a
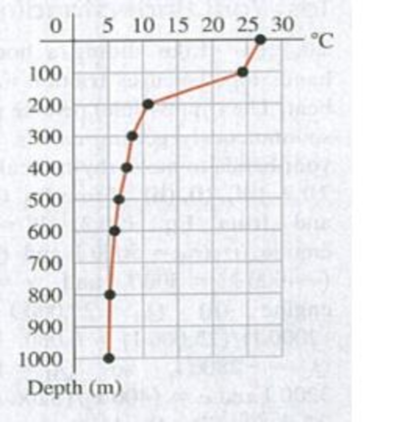
20.61 Compare the entropy change of the warmer water to that of the colder water during one cycle of the heat engine, assuming an ideal Carnot cycle. (a) The entropy does not changc during one cycle in either case. (b) The entropy of both increases, but the entropy of the colder water increases by more because its initial temperature is lower. (c) The entropy of the warmer water decreases by more than the entropy of the colder water increases, because some of the heat removed from the warmer water goes to the work done by the engine. (d) The entropy of the warmer water decreases by the same amount that the entropy of the colder water increases.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
University Physics with Modern Physics (14th Edition)
Additional Science Textbook Solutions
Microbiology with Diseases by Body System (5th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Campbell Biology (11th Edition)
Human Anatomy & Physiology (2nd Edition)
Campbell Essential Biology (7th Edition)
- You are working with a movie director and investigating a scene with a cowboy sliding off a tree limb and falling onto the saddle of a moving horse. The distance of the fall is several meters, and the calculation shows a high probability of injury to the cowboy from the stunt. Let's look at a simpler situation. Suppose the director asks you to have the cowboy step off a platform 2.55 m off the ground and land on his feet on the ground. The cowboy keeps his legs straight as he falls, but then bends at the knees as soon as he touches the ground. This allows the center of mass of his body to move through a distance of 0.660 m before his body comes to rest. (Center of mass will be formally defined in Linear Momentum and Collisions.) You assume this motion to be under constant acceleration of the center of mass of his body. To assess the degree of danger to the cowboy in this stunt, you wish to calculate the average force upward on his body from the ground, as a multiple of the cowboy's…arrow_forwardA box of mass m = 2.00 kg is released from rest at the top of an inclined plane as seen in the figure. The box starts out at height h =0.200 m above the top of the table, the table height is H = 2.00 m, and 0 = 41.0°. H m (a) What is the acceleration (in m/s²) of the box while it slides down the incline? m/s² (b) What is the speed (in m/s) of the box when it leaves the incline? m/s (c) At what horizontal distance (in m) from the end of the table will the box hit the ground? m (d) How long (in s) from when the box is released does it hit the ground? S (e) Does the box's mass affect any of your above answers? Yes Noarrow_forward(a) A sphere made of rubber has a density of 0.940 g/cm³ and a radius of 7.00 cm. It falls through air of density 1.20 kg/m³ and has a drag coefficient of 0.500. What is its terminal speed (in m/s)? m/s (b) From what height (in m) would the sphere have to be dropped to reach this speed if it fell without air resistance? marrow_forward
- The systems shown below are in equilibrium. If the spring scales are calibrated in newtons, what do they read? Ignore the masses of the pulleys and strings and assume the pulleys and the incline are frictionless. (Let m = 2.19 kg and € = 29.0°.) scale in (a) N N scale in (b) scale in (c) N scale in (d) N a C m m m m m b d m Ꮎarrow_forwardAn elevator car has two equal masses attached to the ceiling as shown. (Assume m = 3.10 kg.) m m T₁ T2 (a) The elevator ascends with an acceleration of magnitude 2.00 m/s². What are the tensions in the two strings? (Enter your answers in N.) = N T₁ Τι = N (b) The maximum tension the strings can withstand is 78.8 N. What is the maximum acceleration of the elevator so that a string does not break? (Enter the magnitude in m/s².) m/s²arrow_forward(a) At what speed (in m/s) will a proton move in a circular path of the same radius as an electron that travels at 7.85 x 100 m/s perpendicular to the Earth's magnetic field at an altitude where the field strength is 1.20 x 10-5 T? 4.27e3 m/s (b) What would the radius (in m) of the path be if the proton had the same speed as the electron? 7.85e6 x m (c) What would the radius (in m) be if the proton had the same kinetic energy as the electron? 195.38 x m (d) What would the radius (in m) be if the proton had the same momentum as the electron? 3.7205 marrow_forward
- ! Required information The block shown is made of a magnesium alloy, for which E = 45 GPa and v = 0.35. Know that σx = -185 MPa. NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. 25 mm B D 40 mm 100 mm Determine the magnitude of Oy for which the change in the height of the block will be zero. The magnitude of Oy is MPa.arrow_forwardThe rigid bar ABC is supported by two links, AD and BE, of uniform 37.5 × 6-mm rectangular cross section and made of a mild steel that is assumed to be elastoplastic with E = 200 GPa and σy= 250 MPa. The magnitude of the force Q applied at B is gradually increased from zero to 265 kN and a = 0.640 m. 1.7 m 1 m D A B 2.64 m E Determine the value of the normal stress in each link. The value of the normal stress in link AD is The value of the normal stress in link BE is 250 MPa. MPa.arrow_forwardTwo tempered-steel bars, each 16 in. thick, are bonded to a ½ -in. mild-steel bar. This composite bar is subjected as shown to a centric axial load of magnitude P. Both steels are elastoplastic with E= 29 × 106 psi and with yield strengths equal to 100 ksi and 50 ksi, respectively, for the tempered and mild steel. The load P is gradually increased from zero until the deformation of the bar reaches a maximum value dm = 0.04 in. and then decreased back to zero. Take L = 15 in. NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. 2.0 in. in. 3 in. 3 16 in. Determine the maximum stress in the tempered-steel bars. The maximum stress in the tempered-steel bars is ksi.arrow_forward
- Ammonia enters the compressor of an industrial refrigeration plant at 2 bar, -10°C with a mass flow rate of 15 kg/min and is compressed to 12 bar, 140°C. Heat transfer from the compressor to its surroundings occurs at a rate of 6 kW. For steady-state operation, calculate, (a) the power input to the compressor, in kW, Answer (b) the entropy production rate, in kW/K, for a control volume encompassing the compressor and its immediate surroundings such that heat transfer occurs at 300 K.arrow_forwardNo chatgpt pls will upvotearrow_forwardShown to the right is a block of mass m=5.71kgm=5.71kg on a ramp that makes an angle θ=24.1∘θ=24.1∘ with the horizontal. This block is being pushed by a horizontal force, F=229NF=229N. The coefficient of kinetic friction between the two surfaces is μ=0.51μ=0.51. Enter an expression for the acceleration of the block up the ramp using variables from the problem statement together with gg for the acceleration due to gravity. a=arrow_forward
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





