
(a)
Interpretation:
The synthesis of diene has to be shown from cyclopentanone and acetylene.
Concept introduction:
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid.
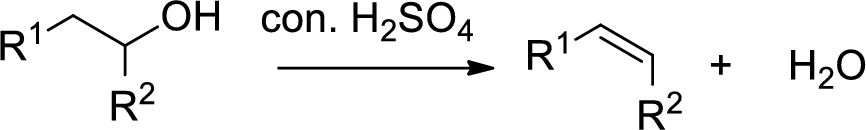
Alcohol is reaction with concentrated sulfuric acid, first alcohol gets protonated forms carbocation (more stable carbocation) followed by elimination of proton (
Tertiary carbocation is more stable than the secondary, secondary carbocation is more stable than primary.
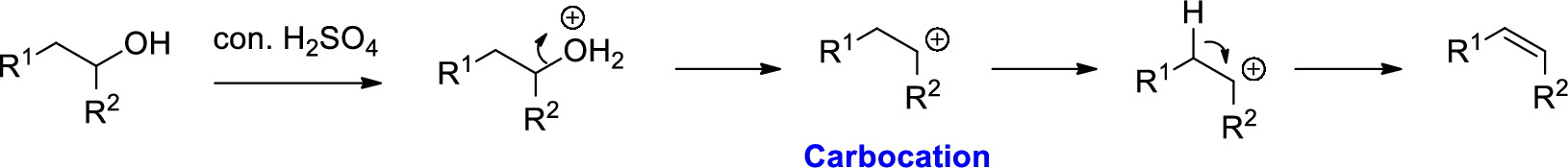
In dehydration reaction, sulfuric acid is act as a proton donor, and which is used to protonate the alcohol and makes carbocation therefore sulfuric acid is the driving force of the reaction. Dehydration reaction will not go without acid (sulfuric acid).
Hydrogenation:
Palladium on carbon (Pd/C) or Pd/ CaCO3 is used as a catalyst for hydrogenation of
(b)
Interpretation:
Stereochemistry of the dicarboxylic acid has to be rationalized.
Concept introduction:
Chiral:
A molecule is non superimposable on its mirror image is called chiral molecule.
Four different atoms attached to a carbon atom is called chiral molecule.
Isomer: A molecule having the same molecular formula but with different chemical structure is called isomer.
Stereoisomers: Stereoisomers are molecules that have the same molecular formula and they differ only in arrangement of atom in three-dimensional space.
Enantiomers: A compound which is non-superimposable mirror image is called enantiomers.
Diastereomers: A compound which is non-superimposable and non-mirror image is called diastereomers.
Racemic mixture: A racemic mixture is simply a mixture containing an equal amount of each enantiomer.
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
Organic Chemistry
- Nonearrow_forwardThe U. S. Environmental Protection Agency (EPA) sets limits on healthful levels of air pollutants. The maximum level that the EPA considers safe for lead air pollution is 1.5 μg/m3 . If your lungs were filled with air containing this level of lead, how many lead atoms would be in your lungs? (Assume a total lung volume of 5.40 Larrow_forwardDuring a(n) ________ process, energy is transferred from the system to the surroundings. exothermic endothermic thermodynamic thermochemical physicalarrow_forward
- Use the following information to determine the enthalpy for the reaction shown below. → S(s) + O2(g) SO2(9) ΔΗ Π ? Reference reactions: S(s) + O2(g) SO3(9) 2SO2(g) + O2(g) → 2SO3(g) AHxn = -395kJ AHrxn = ― -198kJarrow_forwardQ1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardIndicate which of the following is not an element in its standard state at 25oC and 1 atm. Group of answer choices O2(g) H2(g) Ne(g) N(g) C(s, graphite)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





