
Concept explainers
Some Biochemical Reactions of
Alkanes occur naturally in places other than petroleum deposits—in insects, for example. The waxy alkanes dispersed in its cuticle help protect an insect from dehydration. Some insects use volatile alkanes to defend themselves or communicate with others of the same species. Alkanes even serve as starting materials that the insect converts to other biologically important substances.
The major biosynthetic pathway leading to alkanes is by enzyme-catalysed decarboxylation (loss of
Biochemical conversion of alkanes to other substances normally begins with oxidation.
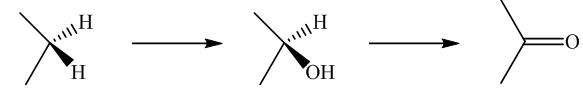
In addition to alkanes, the oxidation of drugs and other substances occurs mainly in the liver and is catalyzed by the enzyme cytochrome
Oxidation by microorganisms has been extensively studied and is often selective for certain kinds of

Female German cockroaches convert the alkane shown to a substance that attracts males.
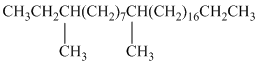
Oxidation at
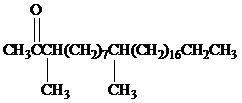
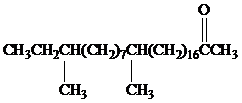
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- Use the average molarity of acetic acid (0.0867M) to calculate the concentration in % (m/v). Then calculate the % difference between the calculated concentrations of your unknown vinegar solution with the 5.00% (w/v%) vinegar solution (check the formula for % difference in the previous lab or online). Before calculating the difference with vinegar, remember that this %(m/v) is of the diluted solution. It has been diluted 10 times.arrow_forwardWhat deprotonates or what can be formed? Please help me understand the problem.arrow_forwardShow work with explanation. Don't give Ai generated solutionarrow_forward
- I have a question about this problem involving mechanisms and drawing curved arrows for acids and bases. I know we need to identify the nucleophile and electrophile, but are there different types of reactions? For instance, what about Grignard reagents and other types that I might not be familiar with? Can you help me with this? I want to identify the names of the mechanisms for problems 1-14, such as Gilman reagents and others. Are they all the same? Also, could you rewrite it so I can better understand? The handwriting is pretty cluttered. Additionally, I need to label the nucleophile and electrophile, but my main concern is whether those reactions differ, like the "Brønsted-Lowry acid-base mechanism, Lewis acid-base mechanism, acid-catalyzed mechanisms, acid-catalyzed reactions, base-catalyzed reactions, nucleophilic substitution mechanisms (SN1 and SN2), elimination reactions (E1 and E2), organometallic mechanisms, and so forth."arrow_forwardSolve the spectroarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forward2. 200 LOD For an unknown compound with a molecular ion of 101 m/z: a. Use the molecular ion to propose at least two molecular formulas. (show your work) b. What is the DU for each of your possible formulas? (show your work) C. Solve the structure and assign each of the following spectra. 8 6 4 2 (ppm) 150 100 50 ō (ppm) 4000 3000 2000 1500 1000 500 HAVENUMBERI-11arrow_forwardComplete the spectroscopy with structurearrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning  Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





