
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 37P
Identify all of the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In the reaction of naphthalene with CrO3 in acetic acid. Indicate
whether a different product is obtained if carried out at 25°C or with
heating (A).
QUESTION: Fill in the answers in the empty green boxes
1. Step 2
2. Step 3
3. Step 4 (SUM)
4. Step 5 (df) (GIVEN)
5. Determine S y/x value
*The data values have been provided in the worksheet attached in the first image*
If the symbol A is placed in a reaction, at what temperature does it take place?
Chapter 2 Solutions
Organic Chemistry
Ch. 2 - PRACTICE PROBLEM
2.1 Cyclobutadiene (below) is...Ch. 2 - Prob. 2PPCh. 2 - Prob. 3PPCh. 2 - Prob. 4PPCh. 2 - Prob. 5PPCh. 2 - Practice Problem 2.6
Using a three-dimensional...Ch. 2 - Practice Problem 2.7
Trichloromethane (, also...Ch. 2 - Prob. 8PPCh. 2 - Prob. 9PPCh. 2 - Practice Problem 2.10
Write bond-line structural...
Ch. 2 - Practice Problem 2.11 Although we shall discuss...Ch. 2 - Practice Problem 2.12 Write bond-line structural...Ch. 2 - Prob. 13PPCh. 2 - Practice Problem 2.14
One way of naming ethers is...Ch. 2 - Practice Problem 2.15 Eugenol is the main...Ch. 2 - Practice Problem 2.16
One way of naming amines is...Ch. 2 - Practice Problem 2.17 Which amines in Practice...Ch. 2 - Prob. 18PPCh. 2 - Prob. 19PPCh. 2 - Practice Problem 2.20
Write bond-line formulas for...Ch. 2 - Practice Problem 2.21
Write bond-line formulas for...Ch. 2 - Practice Problem 2.22
Write bond-line formulas for...Ch. 2 - Prob. 23PPCh. 2 - Practice Problem 2.24 Write another resonance...Ch. 2 - Prob. 25PPCh. 2 - Practice Problem 2.26
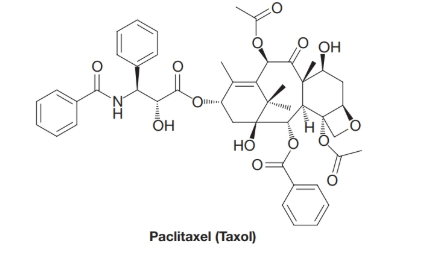
Which compound would you...Ch. 2 - Practice Problem 2.27 Arrange the following...Ch. 2 - Prob. 28PPCh. 2 - Prob. 29PCh. 2 - Identify all of the functional groups in each of...Ch. 2 - 2.31 There are four alkyl bromides with the...Ch. 2 - Prob. 32PCh. 2 - Classify the following alcohols as primary,...Ch. 2 - 2.34 Classify the following amines as primary,...Ch. 2 - Prob. 35PCh. 2 - Identify all of the functional groups in Crixivan,...Ch. 2 - 2.37 Identify all of the functional groups in...Ch. 2 - 2.38 (a) Indicate the hydrophobic and hydrophilic...Ch. 2 - Hydrogen fluoride has a dipole moment of 1.83 D;...Ch. 2 - 2.40 Why does one expect the cis isomer of an...Ch. 2 - Prob. 41PCh. 2 - Prob. 42PCh. 2 - Prob. 43PCh. 2 - 2.44 Consider each of the following molecules in...Ch. 2 - Analyze the statement: For a molecule to be polar,...Ch. 2 - 2.46 Which compound in each of the following...Ch. 2 - Prob. 47PCh. 2 - The IR spectrum of propanoic acid (Fig. 2.16)...Ch. 2 - Prob. 49PCh. 2 - Write structural formulas for four compounds with...Ch. 2 - There are four amides with the formula C3H7NO. (a)...Ch. 2 - Prob. 52PCh. 2 - Prob. 53PCh. 2 - Prob. 54PCh. 2 - Prob. 55PCh. 2 - 2.56 Compound C is asymmetric, has molecular...Ch. 2 - 2.57 Examine the diagram showing an -helical...Ch. 2 - Prob. 1LGPCh. 2 - Prob. 2LGPCh. 2 - Prob. 3LGPCh. 2 - Consider the molecular formula C4H8O2. Predict...Ch. 2 - Consider the molecular formula C4H8O2. If any of...Ch. 2 - Prob. 6LGPCh. 2 - Consider the molecular formula.
7. Pick five...Ch. 2 - Prob. 8LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
What distinguishes the mass spectrum of 2,2-dimethylpropane from the mass spectra of pentane and isopentane?
Organic Chemistry (8th Edition)
A vinyl record is played by rotating the record so that an approximately circular groove in the vinyl slides un...
Fundamentals of Physics Extended
2. The structural and function unit of life is (a) a cell, (b) an organ, (c) the organism, (d) a molecule.
Human Anatomy & Physiology (Marieb, Human Anatomy & Physiology) Standalone Book
Practice Exercise 2
Aspirin is composed of 60.0% carbon, 4.5% hydrogen, and 35.5% oxygen by mass, regardless o...
Chemistry: The Central Science (14th Edition)
8.63 Two flasks of equal volume and at the same temperature contain different gases. One flask contains 10.0 g ...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Two culture media were inoculated with four different bacteria. After incubation, the following results were ob...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- By malonic or acetylacetic synthesis, synthesize 3-methyl-4-oxopentanoic acid (indicate the formulas of the compounds).arrow_forwardoalmitic acid is a 16 carbon acid. In a balanced equation, the products of the sponification of tripalmitin (glyceryl tripalmitate are blank.arrow_forwardWrite the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcoholarrow_forward
- What type of interaction would you expect between the following R groups in the tertiary structure of a protein? O -CH2-CO and -CH2-CH2-CH2-CH2-NH3+ a. disulfide bonds b. salt bridges c. hydrogen bonds HO abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT d. hydrophobic interactions e. peptide bondsarrow_forward4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forwardBy malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forwardDraw the formula for 3-chlorobenzoic acetic anhydride.arrow_forwardBy malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forward
- Obtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forwardEFFICIENTS SAMPLE READINGS CONCENTRATIONS Pigiadient) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) 58 6.274 3.898 301.7 151.2 14150 5.277 3.865 348.9 254.8 B 5.136 3.639 193.7 85.9 605 4.655 3.041 308.6 199.6 05 5.135 3.664 339.5 241.4 0139 4.676 3.662 160.6 87.6 90148 5.086 3.677 337.7 242.5 0092 6.348 3.775 464.7 186.4 PART3 5.081 3.908 223.5 155.8 5.558 3.861 370.5 257.1 4.922 3.66 326.6 242.9 4.752 3.641 327.5 253.3 50 5.018 3.815 336.1 256.0 84 4.959 3.605 317.9 216.6 38 4.96 3.652 203.8 108.7 $3 5.052 3.664 329.8 239.0 17 5.043 3.767 221.9 149.7 052 5.058 3.614 331.7 236.4 5.051 4.005 211.7 152.1 62 5.047 3.637 309.6 222.7 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5 5.033 4.044 334.6 268.7 995 4.706 3.621 305.6 234.4 04 4.816 3.728 340.0 262.7 16 4.828 4.496 304.3 283.2 0.011 4.993 3.865 244.7 143.6 AVERAGE STDEV COUNT 95% CI Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardIf we have two compounds: acetone (CH₃COCH₃) and acetic acid (CH₃COOH), applying heat to them produces an aldol condensation of the two compounds. If this is correct, draw the formula for the final product.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License