
Label each region on the periodic table.
- Noble gases
- Period 3
- Group 4A
- s block elements
- alkaline earth elements
- f block elements
transition metals - group 10
(a)
Interpretation:
Theregion of noble gases on the periodic table is to be labeled.
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in increasing order of atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group.
Answer to Problem 33P
The noble gases are placed on the extreme right column of the periodic table as shown below.
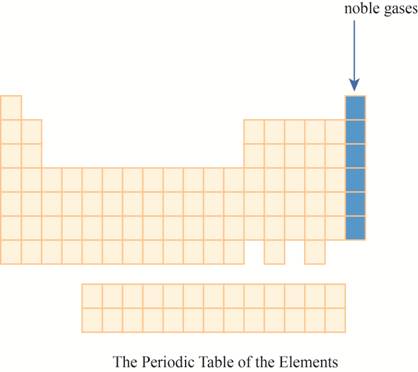
Explanation of Solution
Theelements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell.
The noble gases have their outermost shell fully filled with electrons.Due to fully filled outer atomic orbital, they get the place on extreme right of the periodic table.
The region of noble gases on the periodic table is shown below.
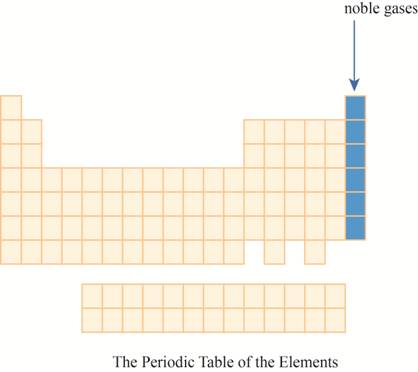
Figure 1
(b)
Interpretation:
The region of period
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in increasing order of atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group.
Answer to Problem 33P
The period
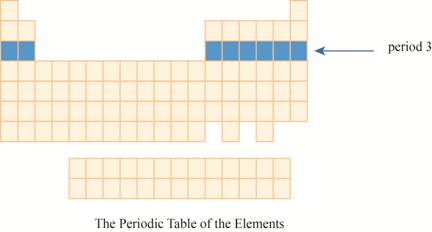
Explanation of Solution
The elements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell. As this outermost shell is filled, the next atom goes to the next row or period.
The third row of the periodic table is known as third period. As the electron goes into third energy level or shell, they are placed in third row. The third shell has two orbitals
These elements are (sodium on the extreme left) to argon (on the extreme right) with atomic numbers,
The region of period
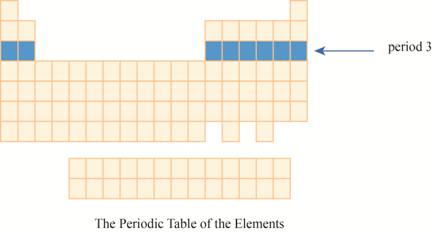
Figure 2
(c)
Interpretation:
The region of group
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in increasing order of atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group
Answer to Problem 33P
The group
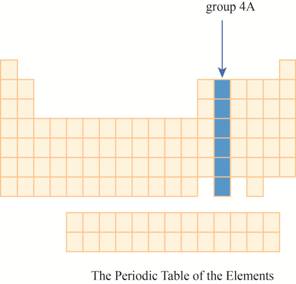
Explanation of Solution
The elements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell. As this outermost shell is filled the next atom goes to the next row or period.
The transition metals are given place in the middle of the periodic table and to its right, main group elements having valence electrons
The group
It has main group elements, carbon and its family. The region of group
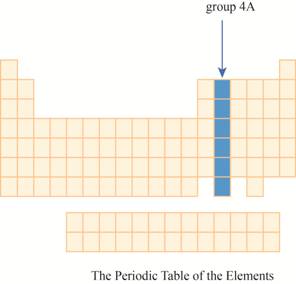
Figure 3
(d)
Interpretation:
The region of
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in an increasing order of the atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group
Answer to Problem 33P
The
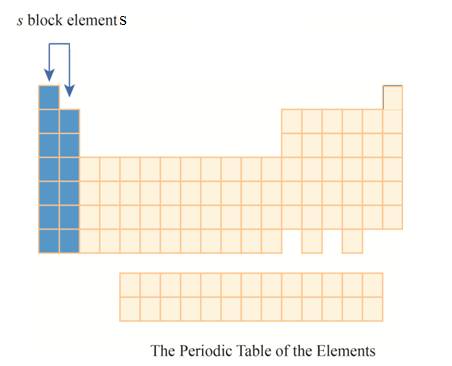
Explanation of Solution
The elements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell. As this outermost shell is filled the next atom goes to the next row or period.
The
The first column has elements with one electron in their outer
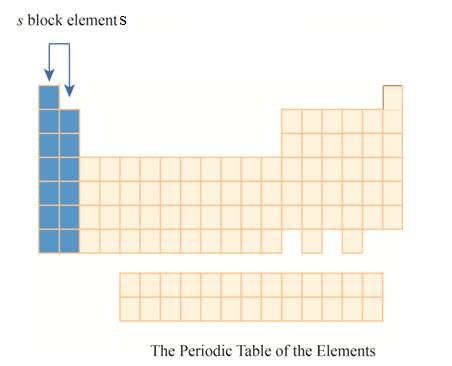
Figure 4
(e)
Interpretation:
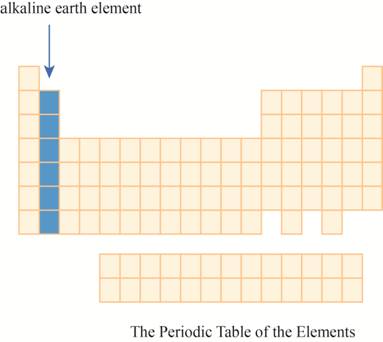
The region of alkaline earth elements on the periodic table is to be labeled.
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in increasing order of atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group
Answer to Problem 33P
The alkaline earth elements are kept in first two columns on the extreme left of periodic tableas shown below.
Explanation of Solution
The elements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell. As this outermost shell is filled the next atom goes to the next row or period.
These elements have two electrons in their outer
The alkaline earth elements are placed in the second columns on the left of periodic table. The elements of this column has electron in their outer
The region of alkaline earth elements on the periodic table are shown below.
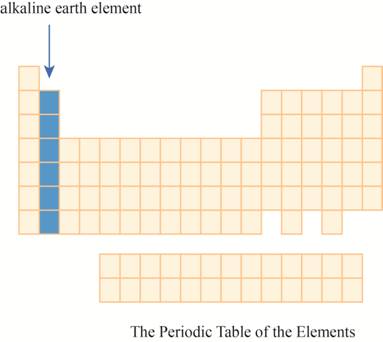
Figure 5
(f)
Interpretation:
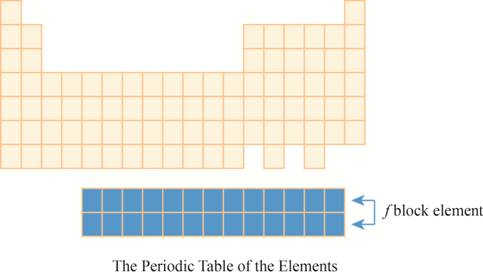
The region of
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in increasing order of atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group
Answer to Problem 33P
The
Explanation of Solution
The elements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell. As this outermost shell is filled the next atom goes to the next row or period.
The
The placement of these elements below the main periodic table is due to their electronic configuration. The
The region of
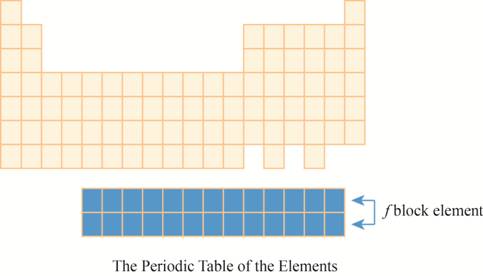
Figure 6
(g)
Interpretation:
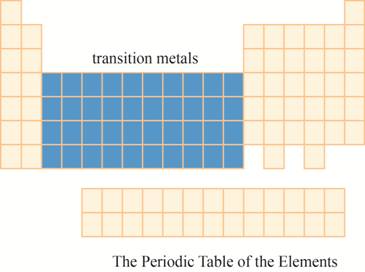
The region of transition metals on the periodic table is to be labeled.
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in increasing order of atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group.
Answer to Problem 33P
The transition metals are kept in ten columns in the middle of the main group elements on the periodic tableas shown below.
Explanation of Solution
The elements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell. As this outermost shell is filled the next atom goes to the next row or period.
In the transition metals the
The region of transition metals on the periodic table is shown below.
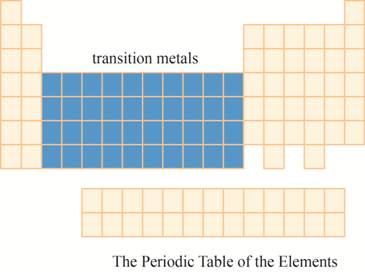
Figure 7
(h)
Interpretation:
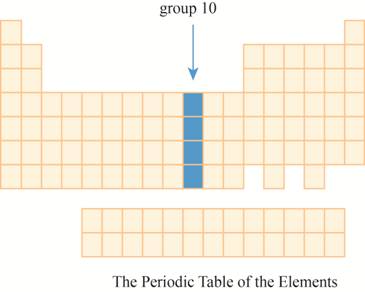
The region group
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in increasing order of atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group.
Answer to Problem 33P
This section has transition element.The region group
Explanation of Solution
The elements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell. As this outermost shell is filled the next atom goes to the next row or period.
The transition elements' inner shell gets filled. They have the electronic configuration of
The group
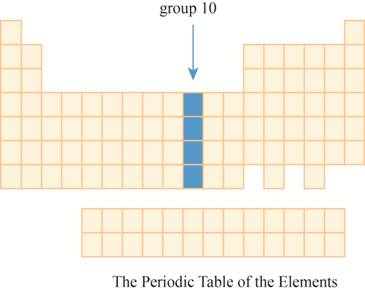
Figure 8
Want to see more full solutions like this?
Chapter 2 Solutions
General, Organic, and Biological Chemistry - 4th edition
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
Organic Chemistry
Fundamentals of Physics Extended
Laboratory Manual For Human Anatomy & Physiology
- Write the calculate the reaction quotient for the following system, if the partial pressure of all reactantsand products is 0.15 atm: NOCl (g) ⇌ NO (g) + Cl2 (g) H = 20.5 kcalarrow_forwardComplete the spectroscopy with structurearrow_forwardcould you answer the questions and draw the complete mechanismarrow_forward
- Complete the spectroscopy with structurearrow_forwardCalculate the reaction quotient for the reaction:NaOH (s) ⇌ Na+ (aq)+ OH- (aq) + 44.4 kJ [Na+] = 4.22 M [OH-] = 6.41 Marrow_forwardGiven the following concentrations for a system, calculate the value for the reaction quotient: Cl2(g)+ CS2(g) ⇌ CCl4(g)+ S2Cl2(g) Cl2 = 31.1 atm CS2 = 91.2 atm CCl4 = 2.12 atm S2Cl2 = 10.4 atmarrow_forward
- Match each chemical or item with the proper disposal or cleanup mwthod, Not all disposal and cleanup methods will be labeled. Metal sheets C, calcium, choroide solutions part A, damp metal pieces Part B, volumetric flask part A. a.Return to correct lables”drying out breaker. Place used items in the drawer.: Rinse with deionized water, dry as best you can, return to instructor. Return used material to the instructor.: Pour down the sink with planty of running water.: f.Pour into aqueous waste container. g.Places used items in garbage.arrow_forwardWrite the equilibrium constant expression for the following reaction: HNO2(aq) + H2O(l) ⇌ H3O+(aq) + NO2-(aq)arrow_forwardWrite the reaction quotient for: Pb2+(aq) + 2 Cl- (aq) ⇌ PbCl2(s)arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





