
Concept explainers
The Dieterici equation of state for one mole of gas is
Where a and b are constants determined experimentally. For NH3(g), a = 10.91 atm. L2 and b = 0.0401 L. Plot the pressure of the gas as the volume of 1.00 mol of NH3(g) expands from 22.4 L to 50.0 L at 273 K, and numerically determine the work done by the gas by measuring the area under the curve.
Interpretation:
The Dieterici equation of state for one mole of gas is
Where a and b are constants determined experimentally. For NH3(g), a = 10.91 atm. L2 and b = 0.0401 L. The pressure of the gas as the volume of 1.00 mol of NH3(g) expands from 22.4 L to 50.0 L at 273 K is to be plotted and numerically the work done by the gas by measuring the area under the curve is to be determined.
Concept introduction:
The ideal gas law considered the molecules of a gas as point particles with perfectly elastic collisions among them in nature. This works importantly well for gases at dilution and at low pressure in many experimental calculations. But the gas molecules are not performing as point masses, and there are situations where the properties of the gas molecules have measurable effect by experiments. Thus, a modification of the ideal gas equation was coined by Johannes D. van der Waals in 1873 to consider size of molecules and the interaction forces among them. Berthelot modified the van der Waals equation as modified Berthelot model of state and further changes was made, and the equation was provided as Dieterici equation of state. The significant advantages of this equation, such as a more realistic critical compressibility factor are documented.
Answer to Problem 2.91E
The Dieterici equation of state for one mole of gas is
Explanation of Solution
The Dieterici equation of state for one mole of gas is
Given,
a = 10.91 atm. L2
b = 0.0401 L
volume of gas initial = 22.4 L
volume of gas final = 50.0 L
temperature of system = 273 K
pressure at 22.4 L is calculated as,
∴ pressure at 22.4 L = 0.9794 atm
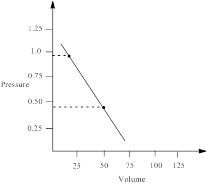
Similarly, pressure at 50 L is calculated as follows;
∴ pressure at 50 L = 0.4435 atm
From the graph the pressure difference can be calculated as;
0.5359 atm.
the work done by the gas by measuring the area under the curve is determined as;
Thus, the pressure of the gas is plotted against volume and the work done in expansion is calculated.
Want to see more full solutions like this?
Chapter 2 Solutions
Bundle: Physical Chemistry, 2nd + Student Solutions Manual
- Indicate whether the following two statements are correct or not:- The S8 heterocycle is the origin of a family of compounds- Most of the elements that give rise to stable heterocycles belong to group d.arrow_forwardcould someone draw curly arrow mechanism for this question pleasearrow_forwardIn the phase diagram of quartz (SiO2), indicate what happens as the pressure increases.arrow_forward
- Show work. Don't give Ai generated solutionarrow_forwardNonearrow_forwardTransmitance 3. Which one of the following compounds corresponds to this IR spectrum? Point out the absorption band(s) that helped you decide. OH H3C OH H₂C CH3 H3C CH3 H3C INFRARED SPECTRUM 0.8- 0.6 0.4- 0.2 3000 2000 1000 Wavenumber (cm-1) 4. Consider this compound: H3C On the structure above, label the different types of H's as A, B, C, etc. In table form, list the labeled signals, and for each one state the number of hydrogens, their shifts, and the splitting you would observe for these hydrogens in the ¹H NMR spectrum. Label # of hydrogens splitting Shift (2)arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





