
The adiabatic exothermic irreversible gas-phase reaction
is to be carried out in a flow reactor for an equimolar feed of A and B. A Levenspiel plot for this reaction is shown in Figure P2-7B.
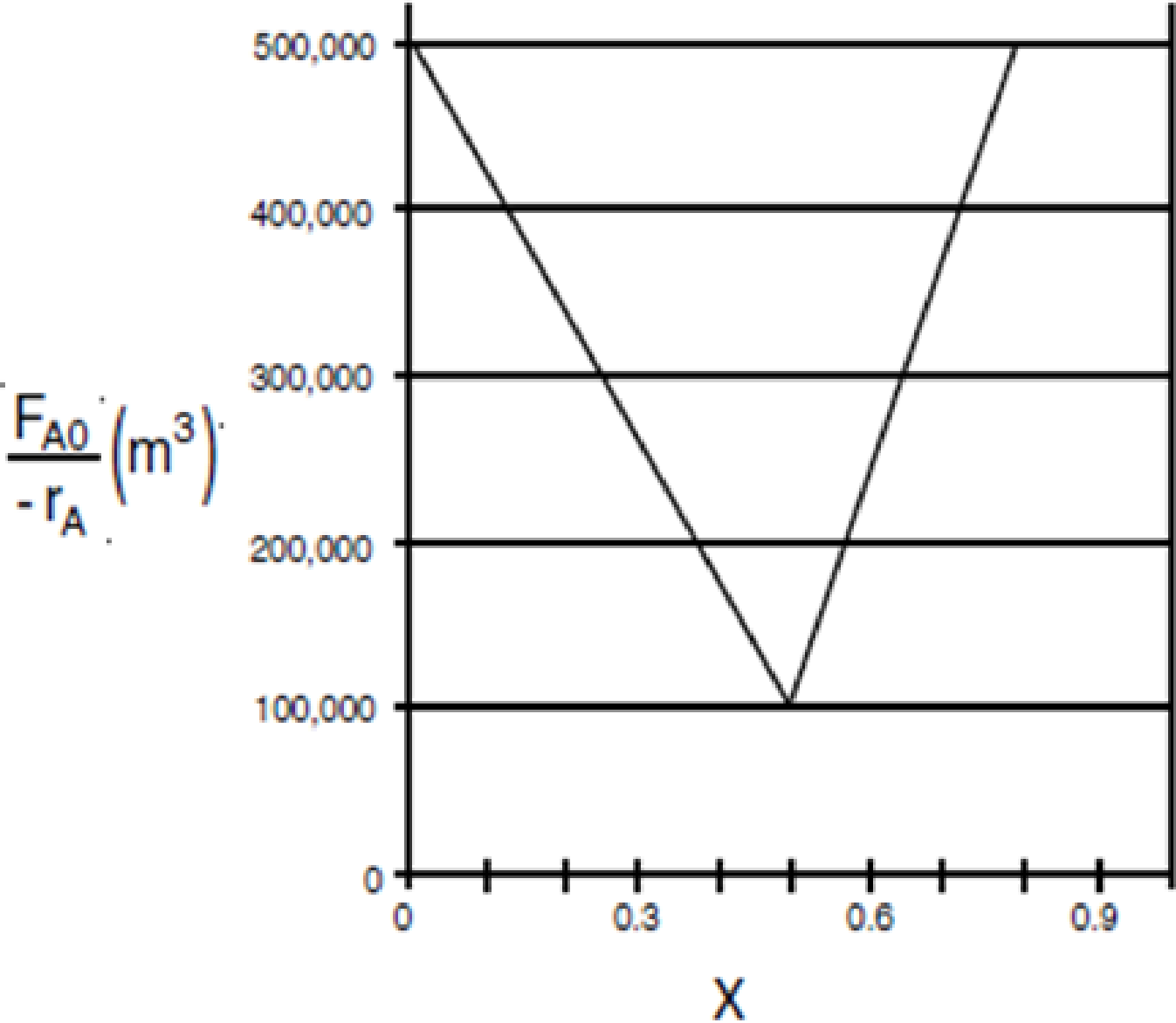
Figure P2-7B Levenspiel plot.
- (a) What PFR volume is necessary to achieve 50% conversion?
- (b) What CSTR volume is necessary to achieve 50% conversion?
- (c) What is the volume of a second CSTR added in series to the first CSTR (Part b) necessary to achieve an overall conversion of 80%?
- (d) What PFR volume must be added to the first CSTR (Part b) to raise the conversion to 80%?
- (e) What conversion can be achieved in a 6 × 104 m3 CSTR? In a 6 × 104 m3 PFR?
- (f) Think critically (cf. Preface, Section I, page xxviii) to critique the answers (numbers) to this problem.
Learn your wayIncludes step-by-step video

Chapter 2 Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Additional Engineering Textbook Solutions
Database Concepts (8th Edition)
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Electric Circuits. (11th Edition)
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Starting Out with Java: From Control Structures through Objects (7th Edition) (What's New in Computer Science)
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
- Derive an expression for incompressible flow in a horizontal pipe of constant diameter andwithout fittings or valves which shows that the pressure is a linear function of pipe length. Whatother assumptions are required for this result? Is this result valid for non-horizontal pipes? Howwill the presence of fittings, valves and other hardware affect this result?arrow_forwardEthylene glycol liquid is used as an antifreeze in many applications. If it is stored in a vessel at a pressure of at 150 psig flows through a ¾ inch-diameter hole to atmospheric pressure. Estimate the discharge rate if the ambient pressure is 1 atm. For ethylene glycol at 77°F, the specific gravity is 1.15 and the viscosity is 25 cP. The molecular weight is 62.07.arrow_forwardPlease help me with parts A through Darrow_forward
- A semi-truck tire is inflated to 110 psig with nitrogen. What will be the initial gas discharge ratein lbm/s due to a 1/16-inch diameter hole? Assume at temperature of 80℉ and an ambientpressure of 1 atm.arrow_forward# 4 The reaction, AB, is to be carried out isothermally in a continuous flow reactor. The entering volumetric flow rate, vo is 10 L/h and is constant (v=vo). Calculate both the CSTR and PFR volumes necessary to reduce the entering concentration of species A from CAD to CA = 0.01 CAO when the entering molar flow rate of species A is 5 mol/h. (a) This reaction is a second order reaction. The reaction rate constant, k is given as 300 L/mol.h. (b) This reaction is a zeroth order reaction. The reaction rate constant, k is given as 0.05 mol/h.L.arrow_forward#3 Using the initial rates method and the given experimental data below to determine the rate law and the value of the rate constant for the reaction, as shown below. All trials are performed at the same temperature. 2NO + Cl2 → 2NCOCI Trial [NO] (mol/L) [Cl₂] (mol/L) Initial rates (mol/L.s) 1 0.10 0.10 0.00300 2 0.10 0.15 0.00450 3 0.15 0.10 0.00675arrow_forward
- #2 The reaction rate constant at temperature, T₁, is 15 mol/L-s while at the reaction rate constant changed to 7 mol/L-s when temperature changed to T2 at 398 K. What is T₁? Given the activation energy is 600 kJ/mol. Assume at this temperature interval, pre-exponential factor and activation energy are constant.arrow_forward#1 Chloral is consumed at a rate of 10 mol/L-s when reacting with chlorobenzene to form DDT and water in the reaction given below. Determine: i) the rate of disappearance of chlorobenzene. ii) the rate of formation of DDT. CCI CHO (Chloral) + 2C6H5Cl (Chlorobenzene) → (C6H4Cl)2CHCCI 3 (DDT) + H2Oarrow_forward#5 The irreversible liquid phase second order reaction, 2A → B, is carried out in a CSTR. The entering concentration of A, CAD is 2 mol/L, and the exit concentration of A, CA is 0.1 mol/L. The volumetric flow rate, vo, is at 3 L/s and is constant (v=vo). The reaction rate constant, k is 0.03 L/mol's. What is the corresponding reactor volume?arrow_forward
- Problem 9.11 An 80 mm long line MN has its end M 15 mm in front of the V.P. The distance between the ends projector is 50 mm. The front view is parallel to and 20 mm above reference line. Draw the projections of the line and determine its inclination with the V.P. Also, locate the traces. Interpretation Front view of a line is parallel to xy, therefore, 1. The line is parallel to the H.P. 2. The top view of the line has true length. 3. The front view has projected length equal to the distance be- tween the projectors. Construction Refer to Fig. 9.11. 1. Draw a reference line xy. Mark point m' 20 mm above xy and point m 15 mm below xy. 2. Draw a 50 mm long line m'n' parallel to xy. 3. Draw an arc with centre m and radius 80 mm to meet projec- tor from point n' at point n. Join mn to represent the top view. Determine its inclination with xy as the inclination of line MN with the V.P. Here = 51°. 4. Traces Extend line mn to meet xy at point v. Project point v to meet m'n' produced at…arrow_forwardoh 30 20 D и D P 60 60 80arrow_forward⑤ b Δε m ab C 40arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





