
Chemistry In Focus
6th Edition
ISBN: 9781305084476
Author: Tro, Nivaldo J., Neu, Don.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2.4YT
Extracting Information from Graphical Data
The following graph shows the average SAT score for incoming students at up- and-coming University.
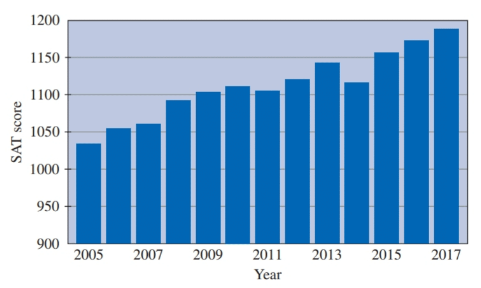
a. What is the total increase in SAT score over this period?
b. What is the average yearly increase in SAT score over this period?
c. What is the total percentage increase in SAT score over this period?
d. What is the average yearly percentage increase over this period?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
>
Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating
the reactants?
esc
?
A
O
O
•If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like.
• If your answer is no, check the box under the drawing area instead.
olo
18
Ar
Explanation
Check
BB
Click and drag to start drawing a structure.
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility
Name the structure
>
For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that:
1. the rate of substitution doesn't depend on nucleophile concentration and
2. the products are a roughly 50/50 mixture of enantiomers.
Substrate A
Substrate B
Faster Rate
X
CI
(Choose one)
(Choose one)
CI
Br
Explanation
Check
Br
(Choose one)
C
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy
A
F10
Chapter 2 Solutions
Chemistry In Focus
Ch. 2 - The volume of a liquid can be measured with a...Ch. 2 - Scientific Notation Express the number 0.0000023...Ch. 2 - Prob. 2SCCh. 2 - Prob. 3SCCh. 2 - Converting Between Units Convert 34.0 cm to...Ch. 2 - Prob. 2.3YTCh. 2 - Extracting Information from Graphical Data The...Ch. 2 - Solving Word Problems...Ch. 2 - Solving Word Problems Involving Units Raised to a...Ch. 2 - Prob. 2.7YT
Ch. 2 - Prob. 2.8YTCh. 2 - Without doing any calculations, determine whether...Ch. 2 - Prob. 1ECh. 2 - Prob. 2ECh. 2 - Prob. 3ECh. 2 - What is the difference between reporting the...Ch. 2 - Prob. 5ECh. 2 - Prob. 6ECh. 2 - Prob. 7ECh. 2 - Prob. 8ECh. 2 - Prob. 9ECh. 2 - What is a conversion factor? Give two examples of...Ch. 2 - Prob. 11ECh. 2 - Identify the decimal part, the exponential part,...Ch. 2 - What is density? Give two examples of possible...Ch. 2 - Since oil floats on water, what can you say about...Ch. 2 - Express each of the following in scientific...Ch. 2 - Express each of the following numbers in...Ch. 2 - Express each of the following in decimal notation:...Ch. 2 - Express each of the following in decimal notation:...Ch. 2 - The circumference of Earth at the equator is...Ch. 2 - The distance from New York to Los Angeles is 2777...Ch. 2 - A can of soda contains 12 fluid ounces. What is...Ch. 2 - A laboratory beaker can hold 150mL. How many fluid...Ch. 2 - A car has a fuel efficiency of 27 miles per...Ch. 2 - A European rental car can travel 17 km on a liter...Ch. 2 - Perform each of the following conversions within...Ch. 2 - Perform each of the following conversions within...Ch. 2 - Prob. 27ECh. 2 - Perform the following conversions between the...Ch. 2 - A pond has a surface area of 1552m2. Convert this...Ch. 2 - An orange has a volume of 54cm3. Convert this...Ch. 2 - Prob. 31ECh. 2 - Prob. 32ECh. 2 - A runner runs at a pace of 8.5 minutes per mile....Ch. 2 - A driver drives an average speed of 58 miles per...Ch. 2 - A sports utility vehicle gets 12 miles per gallon...Ch. 2 - A hybrid electric vehicle (HEV) is a car with both...Ch. 2 - The following graph shows the concentration of an...Ch. 2 - The following graph shows the historical...Ch. 2 - A 28.4-cm3 sample of titanium has a mass of...Ch. 2 - A 1.5-cm3 sample of silicon has a mass of 3.5 g....Ch. 2 - A 5.00-L sample of pure glycerol has a mass of...Ch. 2 - A 3.80-mL sample of mercury has a mass of 51.4g....Ch. 2 - Ethylene glycol (antifreeze) has a density of...Ch. 2 - A thief plans to steal a bar of gold from a womans...Ch. 2 - Prob. 45ECh. 2 - A proton has a radius of approximately 110-13 cm...Ch. 2 - What did Einstein mean when he said, The most...Ch. 2 - Prob. 48ECh. 2 - Prob. 49ECh. 2 - Prob. 50ECh. 2 - Prob. 51ECh. 2 - Consider each of the following balances. Which one...Ch. 2 - Each of the following coins is photographed to...Ch. 2 - Obtain an outdoor thermometer and record the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forwardPredict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forwardDraw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forward
- Predict the major products of this organic reaction: HBr (1 equiv) Δ ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Explanation Check X ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forwardFor the structure below, draw the resonance structure that is indicated by the curved arrow(s). Be sure to include formal charges. :ÖH Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.arrow_forwardUsing the table of Reactants and Products provided in the Hints section, provide the major product (with the correct stereochemistry when applicable) for questions below by selecting the letter that corresponds to the exact chemical structures for the possible product. OH conc Hydrochloric acid 40°C Temp A/arrow_forward
- Using arrows to designate the flow of electrons, complete the reaction below and provide a detailed mechanism for the formation of the product OH conc Hydrochloric acid 40°C Temp All chemical structures should be hand drawn on a piece of paper Paragraph BI UAE +varrow_forwarddraw out the following structures plesearrow_forwardDraw everything on a piece of paper outlining the synthesis from acetaldehyde to 2 cyclopentene carboxaldehyde using carbon based reagants with 3 carbons or fewers. Here is the attached image.arrow_forward
- Manoharan Mariappan, FR.D., 34) Complete the following reaction starting from hex-1-yne proceeding via different substitution reactions forming 2-heptanone. (25 pts). A Sia₂BH H₂O₂ NaOH Br D Mechanism for reaction D - ether-cleavage: 10 B Ph-MgCI, THF H₁₂O+ D HBr (XS) C TsCl, Py CH3-CH2-CH2-ONaarrow_forwardIn the table below, the correct structure for (2R)-3-methylpentan-2-ol (IUPAC name) can be represented by the letter OH OH HE > ' ÕH C B OH D A/ E OHarrow_forwardPredict the major products of the following organic reaction: + A Δ ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Check Click and drag to start drawing a structure. Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

The Creation of Chemistry - The Fundamental Laws: Crash Course Chemistry #3; Author: Crash Course;https://www.youtube.com/watch?v=QiiyvzZBKT8;License: Standard YouTube License, CC-BY