
Concept explainers
Draw the product of each reaction. Use the
a. 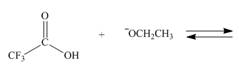 d.
d. 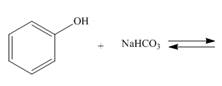
b. 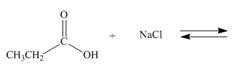 e.
e. ![]()
c. ![]() f.
f. ![]()
(a)
Interpretation: The products of the given reaction are to be drawn. If the equilibrium favors the starting materials or a product is to be predicted.
Concept introduction: According to Bronsted-Lowry theory, when an acid donates a proton the species formed is known as conjugate base and when the base accepts a proton the species formed is known as conjugate acid.
In a reaction which strongly favors the formation of products, the base used to remove proton from the acid should be stronger than the base formed when the proton is removed. In a reaction which favors the products, equilibrium will favors the formation of the weaker acid or weaker base. The
Answer to Problem 2.48P
The products of the given reaction are
Explanation of Solution
The complete reaction between
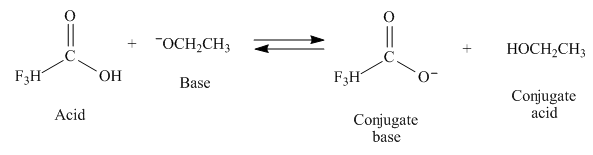
Figure 1
The
The products of the given reaction are
(b)
Interpretation: The products of the given reaction are to be drawn. If the equilibrium favors the starting materials or a product is to be predicted.
Concept introduction: According to Bronsted-Lowry theory, when an acid donates a proton the species formed is known as conjugate base and when the base accepts a proton the species formed is known as conjugate acid.
In a reaction which strongly favors the formation of products, the base used to remove proton from the acid should be stronger than the base formed when the proton is removed. In a reaction which favors the products, equilibrium will favors the formation of the weaker acid or weaker base. The
Answer to Problem 2.48P
The products of the given reaction are
Explanation of Solution
The complete reaction between propanoic acid and sodium chloride is shown below.
The
The products of the given reaction are
(c)
Interpretation: The products of the given reaction are to be drawn. If the equilibrium favors the starting materials or a product is to be predicted.
Concept introduction: According to Bronsted-Lowry theory, when an acid donates a proton the species formed is known as conjugate base and when the base accepts a proton the species formed is known as conjugate acid.
In a reaction which strongly favors the formation of products, the base used to remove proton from the acid should be stronger than the base formed when the proton is removed. In a reaction which favors the products, equilibrium will favors the formation of the weaker acid or weaker base. The
Answer to Problem 2.48P
The products of the given reaction are
Explanation of Solution
The complete reaction between
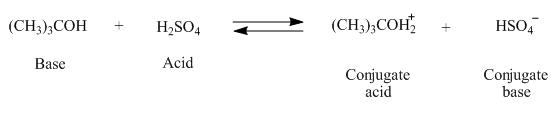
Figure 2
The
The products of the given reaction are
(d)
Interpretation: The products of the given reaction are to be drawn. If the equilibrium favors the starting materials or a product is to be predicted.
Concept introduction: According to Bronsted-Lowry theory, when an acid donates a proton the species formed is known as conjugate base and when the base accepts a proton the species formed is known as conjugate acid.
In a reaction which strongly favors the formation of products, the base used to remove proton from the acid should be stronger than the base formed when the proton is removed. In a reaction which favors the products, equilibrium will favors the formation of the weaker acid or weaker base. Or the
Answer to Problem 2.48P
The products of the given reaction are
Explanation of Solution
The complete reaction between phenol and sodium hydrogen carbonate is shown below.
The
The products of the given reaction are
(e)
Interpretation: The products of the given reaction are to be drawn. If the equilibrium favors the starting materials or a product is to be predicted.
Concept introduction: According to Bronsted-Lowry theory, when an acid donates a proton the species formed is known as conjugate base and when the base accepts a proton the species formed is known as conjugate acid.
In a reaction which strongly favors the formation of products, the base used to remove proton from the acid should be stronger than the base formed when the proton is removed. In a reaction which favors the products, equilibrium will favors the formation of the weaker acid or weaker base. Or the
Answer to Problem 2.48P
The products of the given reaction are
Explanation of Solution
The complete reaction between ethyne and ethyl lithium is shown below.
The
The products of the given reaction are
(f)
Interpretation: The products of the given reaction are to be drawn. If the equilibrium favors the starting materials or a product is to be predicted.
Concept introduction: According to Bronsted-Lowry theory, when an acid donates a proton the species formed is known as conjugate base and when the base accepts a proton the species formed is known as conjugate acid.
In a reaction which strongly favors the formation of products, the base used to remove proton from the acid should be stronger than the base formed when the proton is removed. In a reaction which favors the products, equilibrium will favors the formation of the weaker acid or weaker base. The
Answer to Problem 2.48P
The products of the given reaction are
Explanation of Solution
The complete reaction between methyl amine and
The
The products of the given reaction are
Want to see more full solutions like this?
Chapter 2 Solutions
Organic Chemistry
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





