
Bundle: Introductory Chemistry: An Active Learning Approach, 6th + OWLv2, 1 term (6 months) Printed Access Card
6th Edition
ISBN: 9781305717367
Author: Mark S. Cracolice, Ed Peters
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 2, Problem 2.3TC
Classify the following changes as chemical (C) or physical (P)
a) Baking bread
b) Grinding sugar into powder
c)
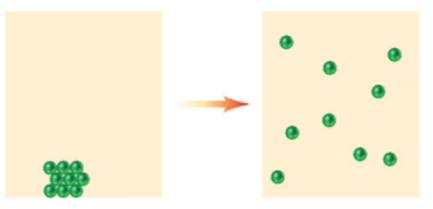
What type of change is represented in c.?
d)
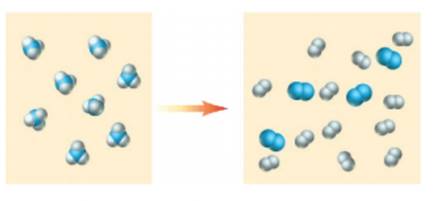
What type of change is represented in d.?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting and don't used Ai solution
Don't used Ai solution and don't used hand raiting
OA. For the structure shown, rank the bond lengths (labeled a, b and c) from shortest to longest. Place your answer in
the box. Only the answer in the box will be graded. (2 points)
H
-CH3
THe
b
Н
Chapter 2 Solutions
Bundle: Introductory Chemistry: An Active Learning Approach, 6th + OWLv2, 1 term (6 months) Printed Access Card
Ch. 2 - Consider the photograph and illustrations of table...Ch. 2 - In the left box, draw a particulate-level...Ch. 2 - Classify the following changes as chemical C or...Ch. 2 - Specific gravity is a physical property. Beakers...Ch. 2 - Classify the following as homogenous or...Ch. 2 - Table salt from the beaker on the left in the...Ch. 2 - Which of the following are compounds, and which...Ch. 2 - Prob. 2.8TCCh. 2 - Identify the net electrical force-attraction,...Ch. 2 - aIs the process of boiling water exothermic or...
Ch. 2 - In everyday language, the term conserve usually...Ch. 2 - Write a brief description of the relationships...Ch. 2 - Write a brief description of the relationships...Ch. 2 - Prob. 3CLECh. 2 - Write a brief description of the relationships...Ch. 2 - Prob. 5CLECh. 2 - Prob. 6CLECh. 2 - Prob. 1ECh. 2 - Classify each of the following as macroscopic,...Ch. 2 - Suggest a reason for studying matter at the...Ch. 2 - How does a chemist think about particles that are...Ch. 2 - Using spheres to represent individual atoms,...Ch. 2 - Describe a piece of ice at the particulate level....Ch. 2 - 7.The word pour is commonly used in reference to...Ch. 2 - Prob. 8ECh. 2 - Which of the three states of matter is most easily...Ch. 2 - Compare the volumes occupied by the same sample of...Ch. 2 - Classify each of the following properties as...Ch. 2 - Classify the italicized property as chemical or...Ch. 2 - Which among the following are physical changes? a...Ch. 2 - Classify each of the following changes as chemical...Ch. 2 - Is the change illustrated below a physical change...Ch. 2 - Is the change in the illustration below a physical...Ch. 2 - Diamonds and graphite are two forms of carbon....Ch. 2 - Aspirin is a pure substance. If you had the choice...Ch. 2 - The substance in the glass below is from a kitchen...Ch. 2 - Are the contents of the bottle in the picture...Ch. 2 - Which of the following particulate illustrations...Ch. 2 - Which of the following particulate illustrations...Ch. 2 - Which of the following are pure substances and...Ch. 2 - Which of the substances below are pure and which...Ch. 2 - Apart from food, list five things in your home...Ch. 2 - Can the terms homogeneous and heterogeneous be...Ch. 2 - Which items in the following list are...Ch. 2 - Classify each of the following mixtures as either...Ch. 2 - Some ice cubes are homogeneous and some are...Ch. 2 - The freshly polished brass cylinder in the picture...Ch. 2 - Draw a particulate-level sketch of a heterogeneous...Ch. 2 - Draw a particulate-level sketch of a homogeneous...Ch. 2 - Suppose someone emptied ball bearings into a...Ch. 2 - Suggest at least two ways to separate ball...Ch. 2 - Prob. 35ECh. 2 - You receive a mixture of table salt and sand and...Ch. 2 - Classify the following as compounds or elements: a...Ch. 2 - Classify each of the following pure substances as...Ch. 2 - Which of the following are elements, and which are...Ch. 2 - Classify each of the following pure substances as...Ch. 2 - Classify each substance in the illustrations below...Ch. 2 - Does each of the particulate-level models below...Ch. 2 - a Which of the following substances would you...Ch. 2 - a Which of the following substances would you...Ch. 2 - Metal A dissolves in nitric acid solution. You can...Ch. 2 - A white, crystalline material that looks like...Ch. 2 - Questions 47 and 48: Samples of matter may be...Ch. 2 - Questions 47 and 48: Samples of matter may be...Ch. 2 - What is the main difference between electrostatic...Ch. 2 - Identify the net electrostatic force attraction,...Ch. 2 - Identify the reactants and products in the...Ch. 2 - In the following equation for a chemical reaction,...Ch. 2 - In the equation Ni+Cu(NO3)2Ni(NO3)2+Cu, which of...Ch. 2 - Write the formulas of the elements that are...Ch. 2 - Prob. 55ECh. 2 - Classify each of the following changes as...Ch. 2 - As a child plays on a swing, at what point in her...Ch. 2 - A bicycle accelerates from 5 miles per hour to 15...Ch. 2 - After solid limestone is heated, the rock that...Ch. 2 - Before electronic flashes were commonly used in...Ch. 2 - The photograph below shows a beaker of water and a...Ch. 2 - Prob. 62ECh. 2 - Prob. 63ECh. 2 - Prob. 64ECh. 2 - Distinguish precisely and in scientific terms the...Ch. 2 - Prob. 66ECh. 2 - A natural-food store advertises that no chemicals...Ch. 2 - Prob. 68ECh. 2 - Name some pure substances you have used today.Ch. 2 - How many homogeneous substances can you reach...Ch. 2 - Which of the following can be pure substances:...Ch. 2 - Can you have a mixture of two elements as well as...Ch. 2 - Can you have more than one compound made of the...Ch. 2 - Rainwater comes from the oceans. Is rainwater more...Ch. 2 - Prob. 75ECh. 2 - Prob. 76ECh. 2 - Consider the sample of matter in the illustration...Ch. 2 - A particulate-level illustration of the reaction...Ch. 2 - Prob. 79ECh. 2 - Prob. 80ECh. 2 - Prob. 81ECh. 2 - Prob. 82ECh. 2 - Particles in the illustration below undergo a...Ch. 2 - Prob. 84E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used hand raitingarrow_forwardQuizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardQ3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. CI Cl H3C-Cl CI a) A B C D Br Br b) A B C Br H3C-Br Darrow_forwardQ4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br .OH Brarrow_forward
- Classify each molecule as optically active or inactive. Determine the configuration at each H соон Chirality center OH 애 He OH H3C Ноос H H COOH A K B.arrow_forwardQ1: Rank the relative nucleophilicity of the following species in ethanol. CH3O¯, CH3OH, CH3COO, CH3COOH, CH3S Q2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- 10. The main product of the following reaction is [1.1:4',1"-terphenyl]-2'-yl(1h-pyrazol-4- yl)methanone Ph N-H Pharrow_forwardDraw the Fischer projection for a D-aldo-pentose. (aldehyde pentose). How many total stereoisomers are there? Name the sugar you drew. Draw the Fischer projection for a L-keto-hexose. (ketone pentose). How many total stereoisomers are there? Draw the enantiomer.arrow_forwardDraw a structure using wedges and dashes for the following compound: H- Et OH HO- H H- Me OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

What are CHNOPS? These Chemical Elements = 98% of Life | Biology | Biochemistry; Author: Socratica;https://www.youtube.com/watch?v=w90wFlR53VM;License: Standard YouTube License, CC-BY