
Concept explainers
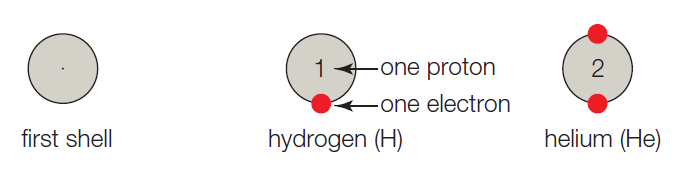
- A. The first shell corresponds to the first energy level, and it can hold up to 2 electrons. Hydrogen has one proton, so it has 1 electron and one vacancy. A helium atom has 2 protons, 2 electrons, and no vacancies.
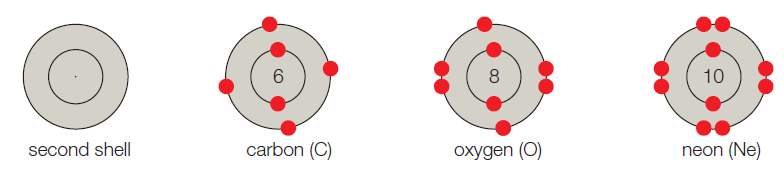
- B. The second shell corresponds to the second energy level, and it can hold up to 8 electrons. Carbon has 6 electrons, so its first shell is full. Its second shell has 4 electrons and four vacancies. Oxygen has 8 electrons and two vacancies. Neon has 10 electrons and no vacancies.
- C. The third shell corresponds to the third energy level, and it can hold up to 8 electrons. A sodium atom has 11 electrons, so its first two shells are full; the third shell has one electron. Thus, sodium has seven vacancies. Chlorine has 17 electrons and one vacancy. Argon has 18 electrons and no vacancies.
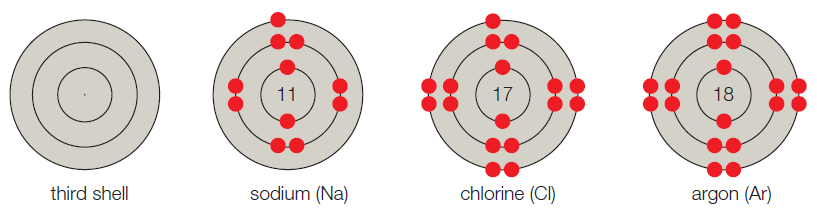
Figure It Out: Which of these models have unpaired electrons in their outer shell?
The atoms are referred to as building blocks of all substances. In other words, it is the basic fundamental unit of the matter. In every atom, the uncharged neutron and positively charged protons are present in the nucleus whereas the negatively charged electron moves around the nucleus. Generally, the shell model system is used to study the valence status of an atom. The atomic number is the number of protons present in the atom which determines the type of the atom
Explanation of Solution
The given shell model consists of about eight atoms namely helium, neon, hydrogen, oxygen, carbon, sodium, chloride, and argon. Of this given atom in the model the hydrogen, carbon, oxygen, chlorine and sodium have unpaired electrons in the outer shell. These unpaired electrons are available to pair with other unpaired electrons in another atom. Thus these atoms combine to form molecules.
Want to see more full solutions like this?
Chapter 2 Solutions
Biology Today and Tomorrow without Physiology (MindTap Course List)
- What is behavioral adaptarrow_forward22. Which of the following mutant proteins is expected to have a dominant negative effect when over- expressed in normal cells? a. mutant PI3-kinase that lacks the SH2 domain but retains the kinase function b. mutant Grb2 protein that cannot bind to RTK c. mutant RTK that lacks the extracellular domain d. mutant PDK that has the PH domain but lost the kinase function e. all of the abovearrow_forwardWhat is the label ?arrow_forward
- Can you described the image? Can you explain the question as well their answer and how to get to an answer to an problem like this?arrow_forwardglg 112 mid unit assignment Identifying melting processesarrow_forwardGive only the mode of inheritance consistent with all three pedigrees and only two reasons that support this, nothing more, (it shouldn't take too long)arrow_forward
- Oarrow_forwardDescribe the principle of homeostasis.arrow_forwardExplain how the hormones of the glands listed below travel around the body to target organs and tissues : Pituitary gland Hypothalamus Thyroid Parathyroid Adrenal Pineal Pancreas(islets of langerhans) Gonads (testes and ovaries) Placentaarrow_forward
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
 Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College





