
Chemistry For Changing Times (14th Edition)
14th Edition
ISBN: 9780321972026
Author: John W. Hill, Terry W. McCreary
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 11RQ
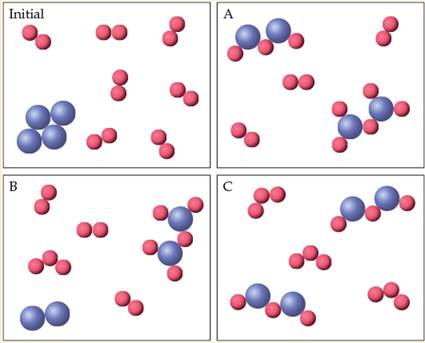
In the figure. the blue spheres represent phosphorus atoms, and the red ones represent oxygen atoms. The box labeled “lnitial represents a mixture. Which one of the other three boxes (A, B. or C) could not represent that mixture after a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
6. Show how you would accomplish the following transformations. (Show the steps and reagents/solvents needed)
2-methylpropene →2,2-dimethyloxiran
I
4) Answer the following exercise with curved arrows indicating who is a
nucleophile or Who is the electrophile?
2.44 Predict the structure of the product formed in the reaction of the organic base
pyridine with the organic acid acetic acid, and use curved arrows to indicate
the direction of electron flow.
7
H3C
OH
N
Pyridine
Acetic acid
Using the data provided please help me answer this question.
Determine the concentration of the iron(Ill) salicylate in the unknown directly from to graph and from the best fit trend-line (least squares analysis) of the graph that yielded a straight line.
Chapter 2 Solutions
Chemistry For Changing Times (14th Edition)
Ch. 2 - Prob. 1RQCh. 2 - Prob. 2RQCh. 2 - Prob. 3RQCh. 2 - Prob. 4RQCh. 2 - Cavendish found that water was composed of two...Ch. 2 - Prob. 6RQCh. 2 - Fructose (fruit sugar) is always composed of 40,0%...Ch. 2 - Outline the main points of Dalton's atomic theory,...Ch. 2 - Prob. 9RQCh. 2 - Prob. 10RQ
Ch. 2 - In the figure. the blue spheres represent...Ch. 2 - 12. a. How is Avogadro’s number linked with the...Ch. 2 - Prob. 13RQCh. 2 - Prob. 14RQCh. 2 - Prob. 15PCh. 2 - 16. An iron nail dissolves in a solution of...Ch. 2 - If you place a 400 g effervescent antacid pill...Ch. 2 - Prob. 18PCh. 2 - 19, Acetylene, used for welding, contains 24.02 g...Ch. 2 - 20. Nitrous oxide (N2O, "laughing gas") contains...Ch. 2 - Prob. 21PCh. 2 - Prob. 22PCh. 2 - Prob. 23PCh. 2 - Prob. 24PCh. 2 - When 18.029 of water is decomposed by...Ch. 2 - Prob. 26PCh. 2 - Prob. 27PCh. 2 - Prob. 28PCh. 2 - Prob. 29PCh. 2 - Prob. 30PCh. 2 - 31. Use Dalton's atomic theory to explain what is...Ch. 2 - Prob. 32PCh. 2 - Hydrogen and oxygen combine in a mass ratio of...Ch. 2 - Prob. 34PCh. 2 - Prob. 35PCh. 2 - A compound containing only oxygen and rubidium has...Ch. 2 - 37. A sample of an oxide of tin with the formula...Ch. 2 - 38. Consider three oxides of nitrogen, X, Y, and...Ch. 2 - Prob. 39PCh. 2 - Prob. 40PCh. 2 - Prob. 41PCh. 2 - Prob. 42PCh. 2 - Prob. 43PCh. 2 - Prob. 44PCh. 2 - A blue solid called azulene is thought to be a...Ch. 2 - Prob. 46PCh. 2 - Prob. 47APCh. 2 - Prob. 48APCh. 2 - Prob. 49APCh. 2 - Prob. 50APCh. 2 - 51. See Table 2.1 . Another compound of nitrogen...Ch. 2 - Prob. 52APCh. 2 - Prob. 53APCh. 2 - Prob. 54APCh. 2 - Prob. 55APCh. 2 - Prob. 56APCh. 2 - Prob. 57APCh. 2 - Prob. 58APCh. 2 - Prob. 2.1CTECh. 2 - When water is electrolyzed, from each one molecule...Ch. 2 - A health-food store has a large display of...Ch. 2 - Prob. 2.4CTECh. 2 - Prob. 2.5CTECh. 2 - Prob. 2.6CTECh. 2 - Prob. 1CGPCh. 2 - Prob. 2CGPCh. 2 - Prob. 3CGPCh. 2 - Prob. 4CGPCh. 2 - Prob. 5CGPCh. 2 - Materials Needed: Alka-Seltzer tablets (8) 1/4 cup...Ch. 2 - Materials Needed: Alka-Seltzer tablets (8) 1/4 cup...Ch. 2 - Prob. 3CHQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me figure out what the slope is and how to calculate the half life Using the data provided.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Follow the curved arrows and draw the structure of the missing reactants, intermediates, or products in the following mechanism. Include all lone pairs. Ignore stereochemistry. Ignore inorganic byproducts. H Br2 (1 equiv) H- Select to Draw Starting Alkene Draw Major Product I I H2O 四: ⑦.. Q Draw Major Charged Intermediate Iarrow_forwardNH (aq)+CNO (aq) → CO(NH2)2(s) Experiment [NH4] (M) [CNO] (M) Initial rate (M/s) 1 0.014 0.02 0.002 23 0.028 0.02 0.008 0.014 0.01 0.001 Calculate the rate contant for this reaction using the data provided in the table.arrow_forward
- 2CIO2 + 20H-1 CIO31 + CIO2 + H2O Experiment [CIO2], M [OH-1], M 1 0.0500 0.100 23 2 0.100 0.100 3 0.100 0.0500 Initial Rate, M/s 0.0575 0.230 0.115 ... Given this date, calculate the overall order of this reaction.arrow_forward2 3 .(be)_[Ɔ+(be)_OI ← (b²)_IƆO+ (be)_I Experiment [1-] M 0.005 [OCI-] 0.005 Initial Rate M/min 0.000275 0.0025 0.005 0.000138 0.0025 0.0025 0.000069 4 0.0025 0.0025 0.000140 Calculate the rate constant of this reaction using the table data.arrow_forward1 2 3 4 I(aq) +OCl(aq) → IO¯¯(aq) + Cl¯(aq) Experiment [I-] M 0.005 [OCI-] 0.005 Initial Rate M/min 0.000275 0.0025 0.005 0.000138 0.0025 0.0025 Calculate the overall order of this reaction using the table data. 0.0025 0.000069 0.0025 0.000140arrow_forward
- H2O2(aq) +3 I¯(aq) +2 H+(aq) → 13(aq) +2 H₂O(l)· ••• Experiment [H2 O2]o (M) [I]o (M) [H+]。 (M) Initial rate (M/s) 1 0.15 0.15 0.05 0.00012 234 0.15 0.3 0.05 0.00024 0.3 0.15 0.05 0.00024 0.15 0.15 0.1 0.00048 Calculate the overall order of this reaction using the table data.arrow_forwardThe U. S. Environmental Protection Agency (EPA) sets limits on healthful levels of air pollutants. The maximum level that the EPA considers safe for lead air pollution is 1.5 μg/m³ Part A If your lungs were filled with air containing this level of lead, how many lead atoms would be in your lungs? (Assume a total lung volume of 5.40 L.) ΜΕ ΑΣΦ = 2.35 1013 ? atoms ! Check your rounding. Your final answer should be rounded to 2 significant figures in the last step. No credit lost. Try again.arrow_forwardY= - 0.039 (14.01) + 0.7949arrow_forward
- Suppose 1.76 g of magnesium acetate (Mg (CH3CO2)2) are dissolved in 140. mL of water. Find the composition of the resulting electrolyte solution. In particular, list the chemical symbols (including any charge) of each dissolved ion in the table below. List only one ion per row. mEq Then, calculate the concentration of each ion in dwrite the concentration in the second column of each row. Be sure you round your answers to the L correct number of significant digits. ion Add Row mEq L x 5arrow_forwardA pdf file of your hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). (A) do the work on a tablet and save as a pdf., it is expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise and every bond that is formed and broken in the process of the reaction, and is expected to see all relevant lone pair electrons and curved arrows.arrow_forwardNonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Atomic Number, Atomic Mass, and the Atomic Structure | How to Pass ChemistryThe Nucleus: Crash Course Chemistry #1; Author: Crash Course;https://www.youtube.com/watch?v=FSyAehMdpyI;License: Standard YouTube License, CC-BY