
(a)
Interpretation:
The possible open-chain structures for the given molecular formula,
Concept Introduction:
Hydrocarbons:
Hydrocarbons contain only carbon and hydrogen atoms which are connected together by covalent bonds such as
Saturated hydrocarbons:
Alkanes are classified under saturated hydrocarbons whose carbon atoms are connected to each other by the carbon-carbon single bonds. The general molecular formula for linear alkanes is
Unsaturated hydrocarbons:
The unsaturated hydrocarbons can be classified further into alkenes and alkynes.
Alkenes:
Alkenes are unsaturated hydrocarbon that must contain atleast one carbon-carbon double bond in it. The general molecular formula for alkenes is
The general structure of alkene is drawn here:
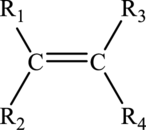
Alkynes:
Alkynes are also unsaturated hydrocarbon that must contain a carbon-carbon triple bond in it. The general molecular formula for linear alkanes is
The general structure of alkyne is shown here:

(b)
Interpretation:
The possible open-chain structures for the given molecular formula,
Concept Introduction:
Refer to part (a).
(c)
Interpretation:
The possible open-chain structures for the given molecular formula,
Concept Introduction:
Refer to part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Foundations of College Chemistry 15e Binder Ready Version + WileyPLUS Registration Card
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardCan you please help me and explain how I would find a mechanism consistent, using my results. Help with number 5.arrow_forward
- The conversion of (CH3)3CI to (CH3)2C=CH2 can occur by either a one-step or a two-step mechanism, as shown in Equations [1] and [2]. [1] + I + H₂Ö: :OH [2] q slow :OH + I¯ H₂Ö: a. What rate equation would be observed for the mechanism in Equation [1]? b. What rate equation would be observed for the mechanism in Equation [2]? c. What is the order of each rate equation (i.e., first, second, and so forth)? d. How can these rate equations be used to show which mechanism is the right one for this reaction? e. Assume Equation [1] represents an endothermic reaction and draw an energy diagram for the reaction. Label the axes, reactants, products, Ea, and AH°. Draw the structure for the transition state. f. Assume Equation [2] represents an endothermic reaction and that the product of the rate-determining step is higher in energy than the reactants or products. Draw an energy diagram for this two-step reaction. Label the axes, reactants and products for each step, and the Ea and AH° for each…arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardFor a complex reaction with the rate equation v = k1[A] + k2[A]2, we can say(A) that it is of order 1.(B) that it is of order 1.5.(C) that it is of order 2.(D) that for certain values of [A] it can behave as if it were of order 1, and for other values as if it were of order 2.arrow_forwarda. Draw a complete arrow pushing mechanism for the following. Is this the thermodynamic or the kinetic product? Use your mechanism to explain your choice. Draw all the resonance. HBr Brarrow_forward
- Which, if any, of the substances had resonance structures? How many resonance structures did each substance have from the following list: CCl4 H2O CO2 C2H4 NH3 SF6 ICl5arrow_forwardSteps and explanation pleasearrow_forwardSteps and explanation please. Add how to solve or target similar problems.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

