
Concept explainers
(a) Infrared spectroscopy provides an easy method for deciding whether the product obtained from the addition of a Grignard reagent to an
(b) How might you follow the rate of the following reaction using UV spectroscopy?
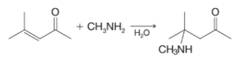
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
ORGANIC CHEMISTRY-ETEXT REG ACCESS
Additional Science Textbook Solutions
Physics for Scientists and Engineers
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Cosmic Perspective Fundamentals
Principles of Anatomy and Physiology
Chemistry: The Central Science (14th Edition)
- Provide the unknown for the given data.arrow_forwardDraw the Lewis structures of two methanol (CH3OH) molecules and depict hydrogenbonding between them with dashed lines. Show all lone pairs. Provide a thorough analysis to apply concept idea into other problems.arrow_forwardSteps and explanation please.arrow_forward
- How could you distinguish between each pair of compounds below using IR? For each pair citeone bond and it’s frequency that you could use to distinguish between them. Please provide thorough analysis to apply into further problems.arrow_forwardSteps and explanation please.arrow_forwardSteps and explanation on how to solve.arrow_forward
- Provide the unknown for the given data.arrow_forwardElectron Arrangement A. Fill in the following chart relating to levels, sublevels and orbitals. Levels (n) 1 Sublevels # of Orbitals per sublevel 2 3 4 # of Electrons per sublevel Total Electrons per level Complete: B. Answer the following questions related to levels, sublevels, orbitals and electrons. 1. How many sublevels are in energy level 2? 2. How many orbitals are in a 4f sublevel? 3. How many electrons can level 3 hold? 4. How many orbitals are in level 4? 5. How many electrons can sublevel 2p hold? 11arrow_forwardProvide the unknown for the given details.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
