
Concept explainers
Answer the following questions about the given triacylglycerol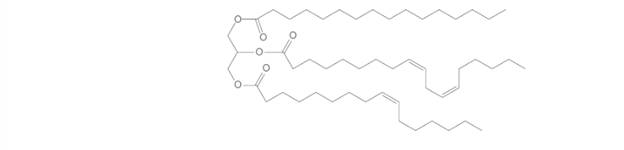
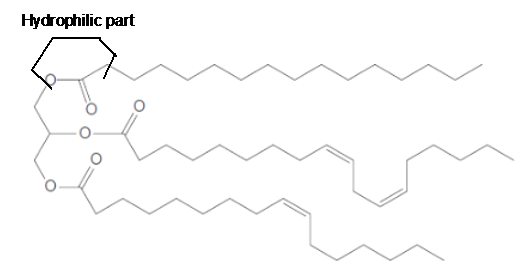
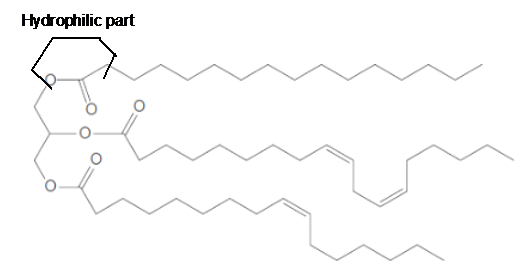
a. What fatty acids are used to form this triacylglycerol?
b. Would you expect this triacylglycerol to be a solid or a liquid at room temperature?
c. What regions are hydrophobic?
d. What regions are hydrophilic?
e. What hydrolysis products are formed when the triacylglycerol is treated with aqueous sulfuric acid?
(a)
Interpretation:
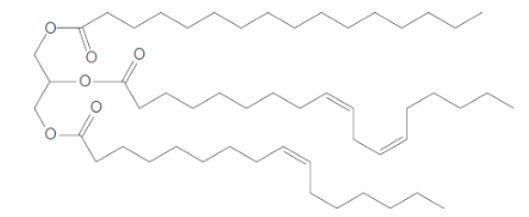
The fatty acids which are used to form the below triacylglycerol needs to be determined.
Concept Introduction:
Lipids are biomolecules that are involved in different biochemical reactions. They are special types of organic molecules which can only be identified with the help of their physical properties, not by the presence of any certain functional group. In general, lipids contain a large number of C-C and C-H bonds with few polar functional groups such as −OH, -SH, etc. For example, triacylglycerol consists of three ester groups whereas Vitamin E is composed of both ether and phenolic groups.
Answer to Problem 50P
In the given triacylglycerol; the fatty acids must be:
Explanation of Solution
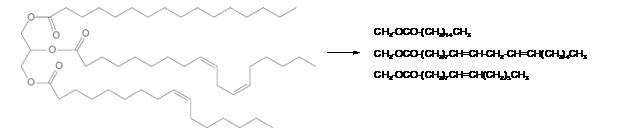
Triacylglycerols are most abundant lipids which are mainly found in animal fat and vegetable oils. They are triesters of glycerol, therefore in the formation of one molecule of triacylglycerol, three molecules of fatty acids react with one molecule of glycerol as given below;
Hence the left side part of hydrocarbon chains is part of fatty acids in the triacylglycerol molecule. The structural formula for the given triacylglycerol is as given below.
In the given triacylglycerol; the fatty acids must be:
(b)
Interpretation:
The physical state of given triacylglycerol needs to be determined.
Concept Introduction:
Lipids are biomolecules that are involved in different biochemical reactions. They are special types of organic molecules which can only be identified with the help of their physical properties, not by the presence of any certain functional group. In general, lipids contain a large number of C-C and C-H bonds with few polar functional groups such as −OH, -SH, etc. For example, triacylglycerol consists of three ester groups whereas Vitamin E is composed of both ether and phenolic groups.
Answer to Problem 50P
The triacylglycerol must be in a liquid state.
Explanation of Solution
In the given triacylglycerol; the fatty acids must be:
Linoleic acid and Palmitoleic acid are unsaturated fatty acids as they have C=C bonds in the long hydrocarbon chain. The unsaturation in fatty acids decreases the melting point of molecule due to bending in the molecule. Since the given triacylglycerol is composed of unsaturated fatty acids, therefore, it should exist in a liquid state.
(c)
Interpretation:
The hydrophobic part of given triacylglycerol needs to be determined.
Concept Introduction:
Lipids are biomolecules that are involved in different biochemical reactions. They are special types of organic molecules which can only be identified with the help of their physical properties, not by the presence of any certain functional group. In general, lipids contain a large number of C-C and C-H bonds with few polar functional groups such as −OH, -SH, etc. For example, triacylglycerol consists of three ester groups whereas Vitamin E is composed of both ether and phenolic groups.
Answer to Problem 50P
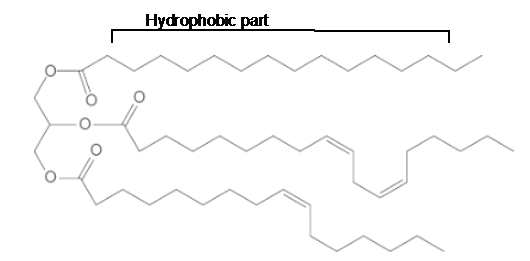
The long hydrocarbon chains in the triacylglycerol molecule is hydrophobic part of the molecule.
Explanation of Solution
Triacylglycerols are most abundant lipids which are mainly found in animal fat and vegetable oils. They are triesters of glycerol, therefore in the formation of one molecule of triacylglycerol, three molecules of fatty acids react with one molecule of glycerol as given below;
The long hydrocarbon chains of the triacylglycerol are hydrophobic part as it is non-polar part.
(d)
Interpretation:
The hydrophilic part of given triacylglycerol needs to be determined.
Concept Introduction:
Lipids are biomolecules that are involved in different biochemical reactions. They are special types of organic molecules which can only be identified with the help of their physical properties, not by the presence of any certain functional group. In general, lipids contain a large number of C-C and C-H bonds with few polar functional groups such as −OH, -SH, etc. For example, triacylglycerol consists of three ester groups whereas Vitamin E is composed of both ether and phenolic groups.
Answer to Problem 50P
The ester linkage in the triacylglycerol molecule is hydrophilic part of the molecule.
Explanation of Solution
Triacylglycerols are most abundant lipids which are mainly found in animal fat and vegetable oils. They are triesters of glycerol, therefore in the formation of one molecule of triacylglycerol, three molecules of fatty acids react with one molecule of glycerol as given below;
The long hydrocarbon chains of the triacylglycerol are hydrophobic part as it is non-polar part whereas the ester linkage in the molecule is hydrophilic part of the molecule.
(e)
Interpretation:
The hydrolysis product of given triacylglycerol with aqueous sulfuric acid needs to be determined.
Concept Introduction:
Lipids are biomolecules that are involved in different biochemical reactions. They are special types of organic molecules which can only be identified with the help of their physical properties, not by the presence of any certain functional group. In general, lipids contain a large number of C-C and C-H bonds with few polar functional groups such as −OH, -SH, etc. For example, triacylglycerol consists of three ester groups whereas Vitamin E is composed of both ether and phenolic groups.
Answer to Problem 50P
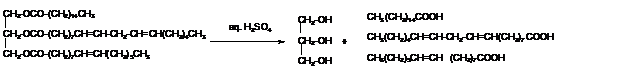
Explanation of Solution
Triacylglycerols are most abundant lipids which are mainly found in animal fat and vegetable oils. They are triesters of glycerol, therefore in the formation of one molecule of triacylglycerol, three molecules of fatty acids react with one molecule of glycerol as given below;
The hydrolysis of triacylglycerol with aqueous sulfuric acid leads to the formation of fatty acid and glycerol molecule.
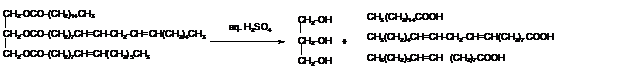
Want to see more full solutions like this?
Chapter 19 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Problem 3-42 Consider 2-methylbutane (isopentane). Sighting along the C2-C3 bond: (a) Draw a Newman projection of the most stable conformation. (b) Draw a Newman projection of the least stable conformation. Problem 3-44 Construct a qualitative potential-energy diagram for rotation about the C-C bond of 1,2-dibromoethane. Which conformation would you expect to be most stable? Label the anti and gauche conformations of 1,2- dibromoethane. Problem 3-45 Which conformation of 1,2-dibromoethane (Problem 3-44) would you expect to have the largest dipole moment? The observed dipole moment of 1,2-dibromoethane is µ = 1.0 D. What does this tell you about the actual conformation of the molecule?arrow_forwardGas Law Studies 1. Mass of zinc Determination of 0.899 2) Moles of zinc 0.01361 mol 3.) Moles of hydrogen 00? ← I was told to calculate this number from mole of zinc. 350m So does that mean it will be 0.01361 mol too? 4 Volume of water collected (mL) 5) VL of water collected (Liters) 0.350 L 6) Temp of water collected (°C) 7) Temp of water collected (°K) 8) Atmospheric pressure (mm) 9) Vapor pressure of water (mm) 10) Corrected pressure of hydrogen 20% 29°C 764.0mm Hg (mm) 17.5mm 11) Corrected pressure of hydrogen (atm) 12) Experimentally calculated value of 19 13. Literature value of R 14) % Error 15) Suggest reasons for the % error (#14)arrow_forwardNo wedge or dashes. Do proper structure. Provide steps and explanation.arrow_forward
- 10 Question (1 point) Draw curved arrow notation to indicate the proton transfer between NaOH and CH3CO₂H. 2nd attempt :0- H See Periodic Table See Hint Draw the products of the proton transfer reaction. Don't add a + sign between the products.arrow_forwardProvide steps and explanation please.arrow_forwardProvide steps to name and label for understanding.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





