
Concept explainers
(a)
Interpretation:
IUPAC name of given below compound has to be given.
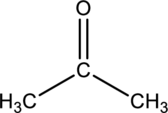
Concept Introduction:
IUAC gives rules for the naming of chemical compounds. These rules are,
In the nomenclature of Ketone, find the longest chain of the compound first then remaining groups and atoms are consider as substituent. In systematic name parent chain is ended with –one suffix.
In the nomenclature, Find the parent chin first then number the carbon atoms in the parent chain by giving lowest number caybonly carbon followed by designate the other substituent in its position in parent chain.
If more than one same substituent or hydroxyl groups were occurs, add a prefix (di-, tri-, tetra-, ect..) in front of parent chain namsse.
Finally the systematic name was written as, the substituent position number with prefix (di-, tri-, tetra-, ect..) of number of substituents followed by name of the substituent then write the parent chain name (which contains large number of carbon atoms in the chain).
(b)
Interpretation:
IUPAC name of given below compound has to be given.
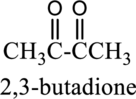
Concept Introduction:
Refer part (a).
(c)
Interpretation:
IUPAC name of given below compound has to be given.
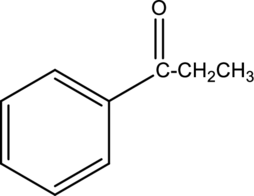
Concept Introduction:
Refer part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
FOUNDATIONS OF COLLEGE CHEM +KNEWTONALTA
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardCan you please help me and explain how I would find a mechanism consistent, using my results. Help with number 5.arrow_forward
- The conversion of (CH3)3CI to (CH3)2C=CH2 can occur by either a one-step or a two-step mechanism, as shown in Equations [1] and [2]. [1] + I + H₂Ö: :OH [2] q slow :OH + I¯ H₂Ö: a. What rate equation would be observed for the mechanism in Equation [1]? b. What rate equation would be observed for the mechanism in Equation [2]? c. What is the order of each rate equation (i.e., first, second, and so forth)? d. How can these rate equations be used to show which mechanism is the right one for this reaction? e. Assume Equation [1] represents an endothermic reaction and draw an energy diagram for the reaction. Label the axes, reactants, products, Ea, and AH°. Draw the structure for the transition state. f. Assume Equation [2] represents an endothermic reaction and that the product of the rate-determining step is higher in energy than the reactants or products. Draw an energy diagram for this two-step reaction. Label the axes, reactants and products for each step, and the Ea and AH° for each…arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardFor a complex reaction with the rate equation v = k1[A] + k2[A]2, we can say(A) that it is of order 1.(B) that it is of order 1.5.(C) that it is of order 2.(D) that for certain values of [A] it can behave as if it were of order 1, and for other values as if it were of order 2.arrow_forwarda. Draw a complete arrow pushing mechanism for the following. Is this the thermodynamic or the kinetic product? Use your mechanism to explain your choice. Draw all the resonance. HBr Brarrow_forward
- Which, if any, of the substances had resonance structures? How many resonance structures did each substance have from the following list: CCl4 H2O CO2 C2H4 NH3 SF6 ICl5arrow_forwardSteps and explanation pleasearrow_forwardSteps and explanation please. Add how to solve or target similar problems.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





