
Concept explainers
Give the number of unpaired electrons in octahedral complexes with strong-field ligands for
(a) Rh3+
(b) Mn3+
(c) Ag+
(d) Pt4+
(e) Au3+
(a)
Interpretation:
The number of unpaired electrons in octahedral complexes with strong field ligands for the metal ion
Concept introduction:
Coordination compounds are those in which a transition metal atom is bonded to a ligand which can be neutral, cation or anion. A transition metal cation has no outer s- electrons available for bonding, rather the inner d -electrons (in case of 3d transition metal elements) are available for making coordinate bonds with the ligands. Electrons are distributed in the five d- orbitals according to Hund’s rule which results in a maximum number of unpaired electrons. The abbreviated electronic configuration of an element depicts the electronic configuration of the elements by making use of noble gas configuration as they have fully-filled electron shells.
Answer to Problem 41QAP
There are zero unpaired electrons in Rh3+.
Explanation of Solution
Rhodium is a 4d transition metal element and its atomic number is 45.Its abbreviated electronic configuration can be written as [Kr] 4d85s1.
When it loses three electrons it leads to the formation of Rh3+ cation, and its abbreviated electronic configuration is written as [Kr] 4d6.
The distribution of electrons in the 4d orbitals when no ligand is present is given as follows:
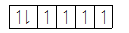
In case of octahedral complexes, the distribution of electrons in the five d-orbitals takes place according to the crystal field theory, according to which in octahedral complexes as the ligand approaches the central metal atom, its d -orbitals get split into lower energy orbitals
Strong field ligands interact directly with the metal d-electrons and causes pairing of the electrons. The compound so formed is a low spin complex.
The distribution of electrons is shown below:
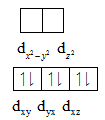
In case of low spin complexes, the unpaired electrons present in the higher energy gets paired with the electrons present in the t2g orbitals. From the above electronic distribution, Rh3+ does not contain any unpaired electrons.
(b)
Interpretation:
The number of unpaired electrons in octahedral complexes with strong field ligands for the metal ion
Concept introduction:
Coordination compounds are those in which a transition metal atom is bonded to a ligand which can be neutral, cation or anion. A transition metal cation has no outer s- electrons available for bonding, rather the inner d -electrons (in case of 3d transition metal elements) are available for making coordinate bonds with the ligands. Electrons are distributed in the five d- orbitals according to Hund’s rule which results in a maximum number of unpaired electrons. The abbreviated electronic configuration of an element depicts the electronic configuration of the elements by making use of noble gas configuration as they have fully-filled electron shells.
Answer to Problem 41QAP
There are two unpaired electrons in Mn3+
Explanation of Solution
Manganese is a 3d transition metal element and its atomic number is 25.Its abbreviated electronic configuration can be written as [Ar] 3d54s2.
When it loses three electrons it leads to the formation of Mn3+ cation, and its abbreviated electronic configuration is written as [Ar] 3d4.
The distribution of electrons in the 3d orbitals when no ligand is present is given as follows:
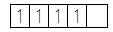
In case of octahedral complexes, the distribution of electrons in the five d-orbitals takes place according to the crystal field theory. According to this, in octahedral complexes as the ligand approaches the central metal atom, its d -orbitals get split into lower energy orbitals
Strong field ligands interact directly with the metal d-electrons and causes pairing of the electrons. The compound so formed is a low spin complex.
The distribution of electrons is shown below:
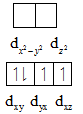
In case of low spin complexes, the unpaired electrons present in the higher energy gets paired with the electrons present in the t2g orbitals. From the above electronic distribution, Mn3+ does not contains two unpaired electrons.
(c)
Interpretation:
The number of unpaired electrons in octahedral complexes with strong field ligands for the metal ion
Concept introduction:
Coordination compounds are those in which a transition metal atom is bonded toa ligand which can be neutral, cation or anion. A transition metal cation has no outer s- electrons available for bonding, rather the inner d -electrons (in case of 3d transition metal elements) are available for making coordinate bonds with the ligands. Electrons are distributed in the five d- orbitals according to Hund’s rule which results in a maximum number of unpaired electrons. The abbreviated electronic configuration of an element depicts the electronic configuration of the elements by making use of noble gas configuration as they have fully-filled electron shells.
Answer to Problem 41QAP
There are zero unpaired electrons in Ag+.
Explanation of Solution
Silver is a 4d transition metal element and its atomic number is 47.Its abbreviated electronic configuration can be written as [Kr] 4d10 5s1.
When it losesone electron it leads to the formation of Ag+ cation, and its abbreviated electronic configuration is written as [Kr] 4d10.
The distribution of electrons in the 4d orbitals when no ligand is present is given as follows:

In case of octahedral complexes, the distribution of electrons in the five d-orbitals takes place according to the crystal field theory according to which, in octahedral complexes, as the ligand approaches the central metal atom, its d -orbitals get split into lower energy orbitals
Strong field ligands interact directly with the metal d-electrons and causes pairing of the electrons. The compound so formed is a low spin complex.
The distribution of electrons is shown below:
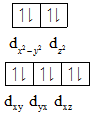
The electrons remain paired in case of strong filed ligands also. From the above electronic distribution, Ag+ does not contain any unpaired electrons.
(d)
Interpretation:
The number of unpaired electrons in octahedral complexes with strong field ligands for the metal ion
Concept introduction:
Coordination compounds are those in which a transition metal atom is bonded toa ligand which can be neutral, cation or anion. A transition metal cation has no outer s- electrons available for bonding, rather the inner d -electrons (in case of 3d transition metal elements) are available for making coordinate bonds with the ligands. Electrons are distributed in the five d- orbitals according to Hund’s rule which results in a maximum number of unpaired electrons. The abbreviated electronic configuration of an element depicts the electronic configuration of the elements by making use of noble gas configuration as they have fully-filled electron shells.
Answer to Problem 41QAP
There are zero unpaired electrons in Pt4+.
Explanation of Solution
In case of transition metal cations, the electrons that are present beyond the noble gas are located in their inner d- orbitals (5d orbitals in case of 5d transition metal elements), this means that they have no outer s- electrons and the distribution of electrons is according to Hund’s rule which states that when orbitals of equal energy are available, then electrons enter singly in the respective orbitals, this gives rise to maximum number of unpaired electrons in transition metal cations.
Platinum is a 5d transition metal element and its atomic number is 78.Its abbreviated electronic configuration can be written as [Xe] 4f145d96s1.
When it losesfour electrons it leads to the formation of Pt4+ cation, and its abbreviated electronic configuration is written as [Xe] 4f14 5d6
The distribution of electrons in the 5d orbitals when no ligand is present is given as follows:

In case of octahedral complexes, the distribution of electrons in the five d-orbitals takes place according to the crystal field theory according to which in octahedral complexes as the ligand approaches the central metal atom its d -orbitals get split into lower energy orbitals
Strong field ligands interact directly with the metal d-electrons and causes pairing of the electrons. The compound so formed is a low spin complex.
The distribution of electrons is shown below:
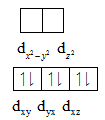
In case of low spin complexes the unpaired electrons present in the higher energy gets paired with the electrons present in the t2g orbitals. From the above electronic distribution, Pt4+ does not contain any unpaired electrons.
(e)
Interpretation:
The number of unpaired electrons in octahedral complexes with strong field ligands for the metal ion
Concept introduction:
Coordination compounds are those in which a transition metal atom is bonded toa ligand which can be neutral, cation or anion. A transition metal cation has no outer s- electrons available for bonding, rather the inner d -electrons (in case of 3d transition metal elements) are available for making coordinate bonds with the ligands. Electrons are distributed in the five d- orbitals according to Hund’s rule which results in a maximum number of unpaired electrons. The abbreviated electronic configuration of an element depicts the electronic configuration of the elements by making use of noble gas configuration as they have fully-filled electron shells.
Answer to Problem 41QAP
There are zero unpaired electrons in Au3+.
Explanation of Solution
Gold is a 5d transition metal element and its atomic number is 79. Its abbreviated electronic configuration can be written as [Xe] 4f14 5d10 6s1.
When it loses three electrons it leads to the formation of Au3+ cation, and its abbreviated electronic configuration is written as [Xe] 4f14 5d8
The distribution of electrons in the 5d orbitals when no ligand is present is given as follows:
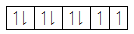
In case of octahedral complexes, the distribution of electrons in the five d-orbitals takes place according to the crystal field theory according to which in octahedral complexes as the ligand approaches the central metal atom its d -orbitals get split into lower energy orbitals
Strong field ligands interact directly with the metal d-electrons and causes pairing of the electrons. The compound so formed is a low spin complex.
The distribution of electrons is shown below:
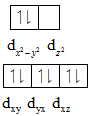
In case of low spin complexes the unpaired electron present in the higher energy
Want to see more full solutions like this?
Chapter 19 Solutions
OWLV2 FOR MASTERTON/HURLEY'S CHEMISTRY:
- Complete boxes in the flow chart. Draw the structure of the organic compound foundin each layer after adding 3M NaOH and extraction. Make sure to include any charges. Provide explanation on answers.arrow_forward== Vid4Q2 Unanswered ☑ Provide IUPAC name of product in the reaction below A 3,4-dimethylcyclohexene B 1,2-dimethylcyclohexane C 1,2-dimethylcyclohexene D 3,4-dimethylcyclohexane H₂ Pdarrow_forward5. Use the MS data to answer the questions on the next page. 14.0 1.4 15.0 8.1 100- MS-IW-5644 26.0 2.8 27.0 6.7 28.0 1.8 29.0 80 4.4 38.0 1.0 39.0 1.5 41.0 1.2 42.0 11.2 43.0 100.0 44.0 4.3 79.0 1.9 80.0 2.6 Relative Intensity 40 81.0 1.9 82.0 2.5 93.0 8.7 20- 95.0 8.2 121.0 2.0 123.0 2.0 136.0 11.8 0 138.0 11.5 20 40 8. 60 a. Br - 0 80 100 120 140 160 180 200 220 m/z Identify the m/z of the base peak and molecular ion. 2 b. Draw structures for each of the following fragments (include electrons and charges): 43.0, 93.0, 95.0, 136.0, and 138.0 m/z. C. Draw a reasonable a-fragmentation mechanism for the fragmentation of the molecular ion to fragment 43.0 m/z. Be sure to include all electrons and formal charges. 6. Using the values provided in Appendix E of your lab manual, calculate the monoisotopic mass for the pyridinium ion (CsH6N) and show your work.arrow_forward
- Nonearrow_forwardStereochemistry: Three possible answers- diastereomers, enantiomers OH CH₂OH I -c=0 21108 1101 41745 HOR CH₂OH IL Но CH₂OH TIL a. Compounds I and III have this relationship with each other: enantiomers b. Compounds II and IV have this relationship with each other: c. Compounds I and II have this relationship with each other: d. *Draw one structure that is a stereoisomer of II, but neither a diastereomer nor an enantiomer. (more than one correct answer)arrow_forwardNonearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





