
Concept explainers
Lactonization Methods
In Section we saw that hydroxy substituted
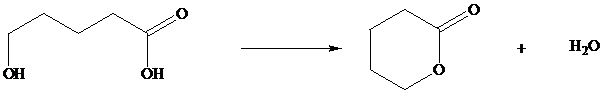
Many natural products are lactones, and chemists have directed substantial attention to developing alternative methods for their synthesis. The most successful of these efforts are based on electrophilic addition to the double bond of unsaturated carboxylic acids. For a generalized electrophilic reagent and, such reactions give a
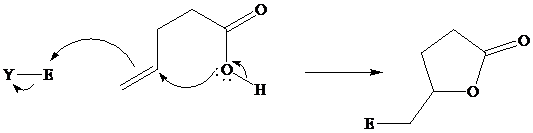
Although the curved arrows show the overall electron flow, the mechanism depends on the electrophilic reagent and normally involves more than one step.
In iodolactonization the electrophilic atom, and represents a source of electrophilic iodine, usually or. In phenylselenolactonization, and is benzeneselenenyl chloride. Anti addition is observed in both iodo andphenylselenolactonization.
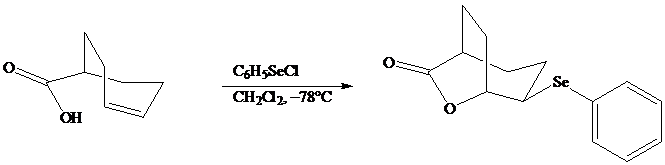
Both iodo andphenylselenolactonization offer the advantage of giving a product containing a
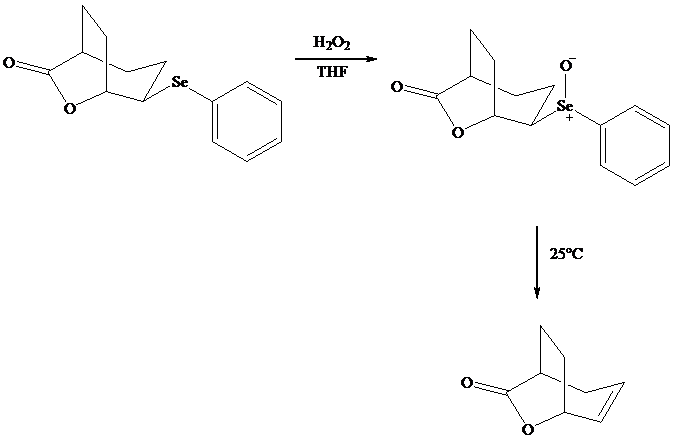
In eliminations of this type, H is always removed from the carbon to selenium that is remote from the lactone oxygen. Elimination is syn.
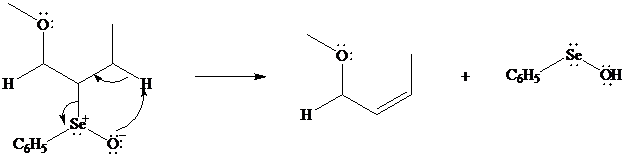
What is the structure of the
(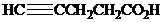 )? Anti addition to the triple bond occurs.
)? Anti addition to the triple bond occurs.
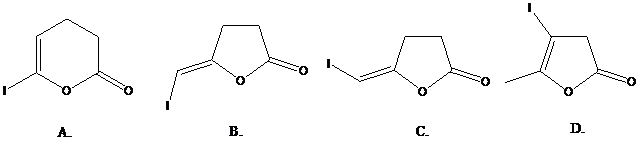
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- Question 16 0/1 pts Choose the correct option for the following cycloaddition reaction. C CF3 CF3 CF3 CF3 The reaction is suprafacial/surafacial and forbidden The reaction is antarafacial/antarafacial and forbidden The reaction is antarafacial/antarafacial and allowed The reaction is suprafacial/surafacial and allowedarrow_forward1. Give the structures of the products obtained when the following are heated. Include stereochemistry where relevant. A NO2 + NO2 B + C N=C CEN + { 2. Which compounds would you heat together in order to synthesize the following?arrow_forwardExplain how myo-inositol is different from D-chiro-inositol. use scholarly sources and please hyperlink.arrow_forward
- What is the molarisuty of a 0.396 m glucose solution if its density is 1.16 g/mL? MM glucose 180.2 /mol.arrow_forwardProvide the proper IUPAC or common name for the following compound. Dashes, commas, and spaces must be used correctly. Br ......Im OHarrow_forwardCan you please help me solve this problems. The top one is just drawing out the skeletal correct and then the bottom one is just very confusing to me and its quite small in the images. Can you enlarge it and explain it to me please. Thank You much (ME EX1) Prblm #33arrow_forward
- I'm trying to memorize VESPR Shapes to solve problems like those. I need help making circles like the second image in blue or using an x- and y-axis plane to memorize these and solve those types of problems, especially the ones given in the top/first image (180, 120, 109.5). Can you help me with this? or is their any other efficient method do soarrow_forwardCan you please explain this problems to me? I'm very confused about it. Please provide a detailed, step-by-step explanation for me! (ME EX1) Prblm 27arrow_forwardQuestion 6 of 7 (1 point) | Question Attempt: 1 of 1 = 1 ✓2 ✓ 3 ✓ 4 ✓ 5 6 ✓ 7 This organic molecule is dissolved in a basic aqueous solution: Jen ✓ ? A short time later sensitive infrared spectroscopy reveals the presence of a new C-OH stretch absorption. That is, must now be a new molecule present with at least one C- OH bond. there 18 In the drawing area below, show the detailed mechanism that could convert the molecule above into the new molecule Ar © + Click and drag to start drawing a structure. Add/Remove step Click and drawing Save For Later Submit Assignmentarrow_forward
- Can you please explain this problem to me? I'm very confused about it. Please provide a detailed, step-by-step explanation for me! (ME EX1) Prblm 22arrow_forwardCan you please explain this problems to me? I'm very confused about it. Please provide a detailed, step-by-step explanation for me! (ME EX1) Prblm 30arrow_forwardThis organic molecule is dissolved in a basic aqueous solution: O ? olo RET A short time later sensitive infrared spectroscopy reveals the presence of a new C-OH stretch absorption. That is, there Ar must now be a new molecule present with at least one C - OH bond. In the drawing area below, show the detailed mechanism that could convert the molecule above into the new molecule. $ Add/Remove steparrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


