
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
4th Edition
ISBN: 9780134110684
Author: Randall D. Knight (Professor Emeritus)
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 19, Problem 20EAP
An experiment measures the temperature of a 500 g substance while steadily supplying heat to it. FIGURE EX19.20 shows the results of the experiment. What are the (a) specific heat of the solid phase, (b) specific heat of the liquid phase, (c) melting and boiling temperatures, and (d) heats of fusion and vaporization?
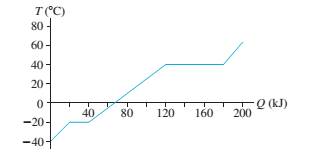
FIGURE EX19.20
.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
SARET CRKS AUTOWAY
12. A stone is dropped from the top of a cliff. It is seen to hit the ground below
after 3.55 s. How high is the cliff?
13. A ball is dropped from rest at the top of a building that is 320 m tall. Assuming
no air resistance, what is the speed of the ball just before it strikes the ground?
14. Estimate (a) how long it took King Kong to fall straight down from the top
of the Empire State Building (280m high), and (b) his velocity just before
"landing".
Useful equations
For Constant Velocity:
V =>
D
X = V₁t + Xo
For Constant Acceleration:
Vr = V + at
X = Xo+Vot +
v=V+2a(X-Xo)
\prom = V +V
V velocity
t = time
D Distance
X = Final Position
Xo Initial Position
V = Final Velocity
Vo Initial Velocity
a = acceleration
For free fall
Yf
= Final Position
Yo Initial Position
g = 9.80
m
$2
For free fall:
V = V + gt
Y=Yo+Vo t +
+gt
V,² = V₁²+2g (Y-Yo)
V+Vo
Vprom=
2
6
Solve the problems
A 11 kg weight is attached to a spring with constant k = 99 N/m and subjected to an external force
F(t) =-704 sin(5t). The weight is initially displaced 4 meters above equilibrium and given an
upward velocity of 5 m/s. Find its displacement for t> 0.
y(t)
ון
Chapter 19 Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Ch. 19 - Prob. 1CQCh. 19 - Do (a) temperature, (b) heat, and (c) thermal...Ch. 19 - Prob. 3CQCh. 19 - You need to raise the temperature of a gas by...Ch. 19 - Prob. 5CQCh. 19 - Prob. 6CQCh. 19 - FIGURE Q19.7 shows two different processes taking...Ch. 19 - FIGURE Q19.8 shows two different processes taking...Ch. 19 - The gas cylinder in FIGURE Q19.9 is a rigid...Ch. 19 - The gas cylinder in FIGURE Q19.10 is well...
Ch. 19 - The gas cylinder in FIGURE Q19.11 is well...Ch. 19 - How much work is done on the gas in the process...Ch. 19 - Prob. 2EAPCh. 19 - Prob. 3EAPCh. 19 - A 2000 cm3 container holds 0.10 mol of helium gas...Ch. 19 - Prob. 5EAPCh. 19 - Prob. 6EAPCh. 19 - Draw a first-law bar chart (see Figure 19.12) for...Ch. 19 - Draw a first-law bar chart (see Figure 19.12) for...Ch. 19 - 9. Draw a first-law bar chart (see Figure 19.12)...Ch. 19 - Prob. 10EAPCh. 19 - J of work are done on a system in a process that...Ch. 19 - How much heat energy must be added to a...Ch. 19 - Prob. 13EAPCh. 19 - Prob. 14EAPCh. 19 - Prob. 15EAPCh. 19 - Prob. 16EAPCh. 19 - One way you keep from overheating is by...Ch. 19 - Prob. 18EAPCh. 19 - Two cars collide head-on while each is traveling...Ch. 19 - An experiment measures the temperature of a 500 g...Ch. 19 - 30 g of copper pellets are removed from a 300°C...Ch. 19 - A 750 g aluminum pan is removed from the stove and...Ch. 19 - A 50.0 g thermometer is used to measure the...Ch. 19 - A 500 g metal sphere is heated to 300°C, then...Ch. 19 - A 65 cm3 block of iron is removed from an 800°C...Ch. 19 - Prob. 26EAPCh. 19 - A container holds 1.0 g of oxygen at a pressure of...Ch. 19 - The volume of a gas is halved during an adiabatic...Ch. 19 - Prob. 29EAPCh. 19 - Prob. 30EAPCh. 19 - Prob. 31EAPCh. 19 - Prob. 32EAPCh. 19 - Prob. 33EAPCh. 19 - Prob. 34EAPCh. 19 - Prob. 35EAPCh. 19 - What maximum power can be radiated by a...Ch. 19 - Radiation from the head is a major source of heat...Ch. 19 - Prob. 38EAPCh. 19 - Prob. 39EAPCh. 19 - Prob. 40EAPCh. 19 - Prob. 41EAPCh. 19 - Prob. 42EAPCh. 19 - Prob. 43EAPCh. 19 - The specific heat of most solids is nearly...Ch. 19 - Prob. 45EAPCh. 19 - Prob. 46EAPCh. 19 - Prob. 47EAPCh. 19 - Prob. 48EAPCh. 19 - .0 mol of gas are at 30°C and a pressure of 1.5...Ch. 19 - A 6.0-cm-diameter cylinder of nitrogen gas has a...Ch. 19 - Prob. 51EAPCh. 19 - An ideal-gas process is described by p = cV 1/2 ,...Ch. 19 - Prob. 53EAPCh. 19 - Prob. 54EAPCh. 19 - Prob. 55EAPCh. 19 - Prob. 56EAPCh. 19 - Prob. 57EAPCh. 19 - .10 mol of nitrogen gas follow the two processes...Ch. 19 - Prob. 59EAPCh. 19 - Prob. 60EAPCh. 19 - Prob. 61EAPCh. 19 - Prob. 62EAPCh. 19 - Prob. 63EAPCh. 19 - Prob. 64EAPCh. 19 - Prob. 65EAPCh. 19 - Prob. 66EAPCh. 19 - Prob. 67EAPCh. 19 - Prob. 68EAPCh. 19 - Prob. 69EAPCh. 19 - A cylindrical copper rod and an iron rod with...Ch. 19 - Prob. 71EAPCh. 19 - Prob. 72EAPCh. 19 - Prob. 73EAPCh. 19 - Prob. 74EAPCh. 19 - Prob. 75EAPCh. 19 - Prob. 76EAPCh. 19 - Prob. 77EAPCh. 19 - Prob. 78EAPCh. 19 - Prob. 79EAPCh. 19 - Prob. 80EAPCh. 19 - Prob. 81EAPCh. 19 - Prob. 82EAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 7. A race car accelerates from rest to 55 m s-1 in 5.0 seconds. The acceleration of the car Is m s-² 8. An object's speed increases uniformly from 10.5 km per hour to 99.8 km per hour in 2.41 seconds. Calculate the acceleration in m s-2 and express your answer to three significant figures. 9. The acceleration-time graph of a car is shown below. The initial speed of the car is 5.0 m s-1. # Acceleration (ms) 12 8.0- 4.0- 2.0 4.0 6.0 Time (s) Calculate the velocity of the car at t = 4.0 s. 3arrow_forwardNo chatgpt pls will upvotearrow_forwardNo chatgpt pls will upvotearrow_forward
- Problem Seven. A football receiver running straight downfield at 5.60 m/s is 11.5 m in front of the quarterback when a pass is thrown downfield at an angle of 35.0° horizon. above the 8.) If the receiver never changes speed and the ball is caught at the same height from which it was thrown, find the distance between the quarterback and the receiver when the catch is made. (A) 21.3 (B) 17.8 (C) 18.8 (D) 19.9 (E) 67.5arrow_forwardPlease solve and answer the question correctly please. Thank you!!arrow_forwardPlease solve and answer the question correctly please. Thank you!!arrow_forward
- Please view both photos, and answer the question correctly please. Thank you!!arrow_forwardA thrown brick hits a window, but doesn't break it. Instead it reverses direction and ends down on the ground below the window. Since the brick didn't break the glass, we know: О The force of the brick on the glass > the force of the glass on the brick. О The force of the brick on the glass the force of the glass on the brick. = О The force of the brick on the glass < the force of the glass on the brick. О The brick didn't slow down as it broke the glass.arrow_forwardAlexandra (wearing rubber boots for traction) is attempting to drag her 32.6-kg Golden Retriever across the smooth ice by applying a horizontal force. What force must she apply to move the dog with a constant speed of 0.950 m/s? ☐ 31.0 lb. ☐ 319 kg. ○ Zero. 32.6 kg.arrow_forward
- The figure shows a graph of the acceleration of an object as a function of the net force acting on it. The mass of this object, in grams, is closest to 11 a(m/s²) 8.0+ 6.0- 4.0- 2.0- 0+ F(N) 0.00 0.50 1.00 ☐ 130 ○ 8000 ☐ 89arrow_forwardValues that are within standard deviations represent measurements that are considered to be near the true value. Review the data from the lab and determine whether your data is within standard deviations. Report, using numerical values, whether your data for each angle is within standard deviations. An acceptable margin of error typically falls between 4% and 8% at the 95% confidence level. Review your data for each angle to determine whether the margin of error is within an acceptable range. Report with numerical values, whether your data for each angle is within an acceptable margin of error. Can you help explain what my data means in terms of the standard deviation and the ME? Thanks!arrow_forwardA sinusoidal wave is propagating along a stretched string that lies along the x-axis. The displacement of the string as a function of time is graphed in (Figure 1) for particles at x = 0 and at x = 0.0900 m. You are told that the two points x = 0 and x = 0.0900 m are within one wavelength of each other. If the wave is moving in the +x-direction, determine the wavelength. If instead the wave is moving in the -x-direction, determine the wavelength. Please show all stepsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY