
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
4th Edition
ISBN: 9780134110684
Author: Randall D. Knight (Professor Emeritus)
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 19, Problem 7CQ
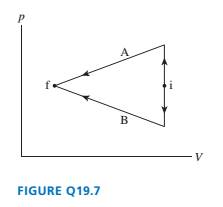
FIGURE Q19.7 shows two different processes taking an ideal gas
from state i to state f. Is the work done on the gas in process A
greater than, less than, or equal to the work done in process B?
Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4
Problem 4) A particle is being pushed up a smooth slot by a rod. At the instant when 0 = rad,
the angular speed of the arm is ė = 1 rad/sec, and the angular acceleration is = 2 rad/sec².
What is the net force acting on the 1 kg particle at this instant? Express your answer as a vector
in cylindrical coordinates. Hint: You can express the radial coordinate as a function of the angle
by observing a right triangle. (20 pts)
Ꮎ
2 m
Figure 3: Particle pushed by rod along vertical path.
4
Problem 4) A particle is being pushed up a smooth slot by a rod. At the instant when 0 = rad,
the angular speed of the arm is ė = 1 rad/sec, and the angular acceleration is = 2 rad/sec².
What is the net force acting on the 1 kg particle at this instant? Express your answer as a vector
in cylindrical coordinates. Hint: You can express the radial coordinate as a function of the angle
by observing a right triangle. (20 pts)
Ꮎ
2 m
Figure 3: Particle pushed by rod along vertical path.
please solve and answer the question correctly. Thank you!!
Chapter 19 Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Ch. 19 - Prob. 1CQCh. 19 - Do (a) temperature, (b) heat, and (c) thermal...Ch. 19 - Prob. 3CQCh. 19 - You need to raise the temperature of a gas by...Ch. 19 - Prob. 5CQCh. 19 - Prob. 6CQCh. 19 - FIGURE Q19.7 shows two different processes taking...Ch. 19 - FIGURE Q19.8 shows two different processes taking...Ch. 19 - The gas cylinder in FIGURE Q19.9 is a rigid...Ch. 19 - The gas cylinder in FIGURE Q19.10 is well...
Ch. 19 - The gas cylinder in FIGURE Q19.11 is well...Ch. 19 - How much work is done on the gas in the process...Ch. 19 - Prob. 2EAPCh. 19 - Prob. 3EAPCh. 19 - A 2000 cm3 container holds 0.10 mol of helium gas...Ch. 19 - Prob. 5EAPCh. 19 - Prob. 6EAPCh. 19 - Draw a first-law bar chart (see Figure 19.12) for...Ch. 19 - Draw a first-law bar chart (see Figure 19.12) for...Ch. 19 - 9. Draw a first-law bar chart (see Figure 19.12)...Ch. 19 - Prob. 10EAPCh. 19 - J of work are done on a system in a process that...Ch. 19 - How much heat energy must be added to a...Ch. 19 - Prob. 13EAPCh. 19 - Prob. 14EAPCh. 19 - Prob. 15EAPCh. 19 - Prob. 16EAPCh. 19 - One way you keep from overheating is by...Ch. 19 - Prob. 18EAPCh. 19 - Two cars collide head-on while each is traveling...Ch. 19 - An experiment measures the temperature of a 500 g...Ch. 19 - 30 g of copper pellets are removed from a 300°C...Ch. 19 - A 750 g aluminum pan is removed from the stove and...Ch. 19 - A 50.0 g thermometer is used to measure the...Ch. 19 - A 500 g metal sphere is heated to 300°C, then...Ch. 19 - A 65 cm3 block of iron is removed from an 800°C...Ch. 19 - Prob. 26EAPCh. 19 - A container holds 1.0 g of oxygen at a pressure of...Ch. 19 - The volume of a gas is halved during an adiabatic...Ch. 19 - Prob. 29EAPCh. 19 - Prob. 30EAPCh. 19 - Prob. 31EAPCh. 19 - Prob. 32EAPCh. 19 - Prob. 33EAPCh. 19 - Prob. 34EAPCh. 19 - Prob. 35EAPCh. 19 - What maximum power can be radiated by a...Ch. 19 - Radiation from the head is a major source of heat...Ch. 19 - Prob. 38EAPCh. 19 - Prob. 39EAPCh. 19 - Prob. 40EAPCh. 19 - Prob. 41EAPCh. 19 - Prob. 42EAPCh. 19 - Prob. 43EAPCh. 19 - The specific heat of most solids is nearly...Ch. 19 - Prob. 45EAPCh. 19 - Prob. 46EAPCh. 19 - Prob. 47EAPCh. 19 - Prob. 48EAPCh. 19 - .0 mol of gas are at 30°C and a pressure of 1.5...Ch. 19 - A 6.0-cm-diameter cylinder of nitrogen gas has a...Ch. 19 - Prob. 51EAPCh. 19 - An ideal-gas process is described by p = cV 1/2 ,...Ch. 19 - Prob. 53EAPCh. 19 - Prob. 54EAPCh. 19 - Prob. 55EAPCh. 19 - Prob. 56EAPCh. 19 - Prob. 57EAPCh. 19 - .10 mol of nitrogen gas follow the two processes...Ch. 19 - Prob. 59EAPCh. 19 - Prob. 60EAPCh. 19 - Prob. 61EAPCh. 19 - Prob. 62EAPCh. 19 - Prob. 63EAPCh. 19 - Prob. 64EAPCh. 19 - Prob. 65EAPCh. 19 - Prob. 66EAPCh. 19 - Prob. 67EAPCh. 19 - Prob. 68EAPCh. 19 - Prob. 69EAPCh. 19 - A cylindrical copper rod and an iron rod with...Ch. 19 - Prob. 71EAPCh. 19 - Prob. 72EAPCh. 19 - Prob. 73EAPCh. 19 - Prob. 74EAPCh. 19 - Prob. 75EAPCh. 19 - Prob. 76EAPCh. 19 - Prob. 77EAPCh. 19 - Prob. 78EAPCh. 19 - Prob. 79EAPCh. 19 - Prob. 80EAPCh. 19 - Prob. 81EAPCh. 19 - Prob. 82EAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- No chatgpt plsarrow_forwardNo chatgpt pls will upvotearrow_forwardA shot putter releases a shot at 13 m/s at an angle of 42 degrees to the horizontal and from a height of 1.83 m above the ground. Calculate. Note: For each question draw a diagram to show the vector/s. Show all the steps and provide units in the answers. Provide answer to 2 decimal places unless stated otherwise. Answer all parts and show all work please.arrow_forward
- A player kicks a football at the start of the game. After a 4 second flight, the ball touches the ground 50 m from the kicking tee. Assume air resistance is negligible and the take-off and landing height are the same (i.e., time to peak = time to fall = ½ total flight time). Calculate: Note: For each question draw a diagram to show the vector/s. Show all the step and provide units in the answers. Provide answer to 2 decimal places unless stated otherwise.)arrow_forwardIf I stand next to a wall on a frictionless skateboard and push the wall with a force of 25 N, what would my acceleration be if my mass is 75 kg?arrow_forwardWhat is the direction of the magnetic force on the current shown in the following figures?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY