
Organic Chemistry, Loose-leaf Version
8th Edition
ISBN: 9781305865549
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 19, Problem 19.69P
Interpretation Introduction
Interpretation:
The synthesis of tranquilizer volnoctamide has to be given using diethyl malonate as the source of the carboxamide group.
Concept Introduction:
Acid chlorides are most often prepared by treating a
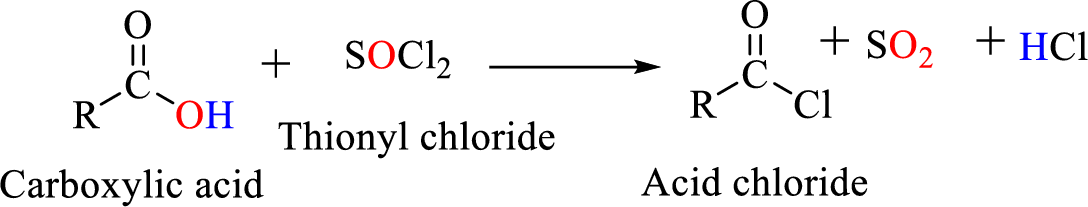
Reaction of acid chloride with ammonia or
The reaction of acid chlorides with ammonia and primary and secondary amines will yield amide.
The nucleophilic addition of ammonia or amine to the carbonyl carbon occurs which is followed by a proton transfer forming an addition intermediate that will eliminate chloride and lose a proton to give the amide compound.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please sirrr soollveee these parts pleaseeee and thank youuuuu
Please sirrr soollveee these parts pleaseeee and thank youuuuu, don't solve it by AI plleeaasseee
Please sirrr soollveee these parts pleaseeee and thank youuuuu
Chapter 19 Solutions
Organic Chemistry, Loose-leaf Version
Ch. 19.2 - Prob. 19.1PCh. 19.2 - Prob. 19.2PCh. 19.2 - Prob. 19.3PCh. 19.3 - Prob. 19.4PCh. 19.3 - Prob. 19.5PCh. 19.3 - Prob. 19.6PCh. 19.5 - Prob. 19.7PCh. 19.5 - Prob. 19.8PCh. 19.5 - Prob. 19.9PCh. 19.6 - Prob. 19.10P
Ch. 19.6 - Prob. 19.11PCh. 19.7 - Prob. 19.12PCh. 19.8 - Prob. 19.13PCh. 19.8 - Prob. 19.14PCh. 19.8 - Prob. 19.15PCh. 19.8 - Prob. 19.16PCh. 19.9 - Prob. 19.17PCh. 19.9 - Prob. AQCh. 19.9 - Prob. BQCh. 19.9 - Prob. CQCh. 19.9 - Prob. DQCh. 19.9 - Prob. EQCh. 19.9 - Prob. FQCh. 19.9 - Prob. GQCh. 19.9 - Intermediate G in Synthesis III is produced as a...Ch. 19.9 - Prob. IQCh. 19.9 - Prob. JQCh. 19 - Prob. 19.18PCh. 19 - Prob. 19.19PCh. 19 - Prob. 19.20PCh. 19 - Prob. 19.21PCh. 19 - Prob. 19.22PCh. 19 - Prob. 19.23PCh. 19 - Cyclohexene can be converted to...Ch. 19 - Prob. 19.25PCh. 19 - Prob. 19.26PCh. 19 - Prob. 19.27PCh. 19 - Prob. 19.28PCh. 19 - Prob. 19.29PCh. 19 - Prob. 19.30PCh. 19 - Draw structural formulas for the -ketoesters...Ch. 19 - Prob. 19.32PCh. 19 - Prob. 19.33PCh. 19 - Propose a synthesis for each ketone, using as one...Ch. 19 - Prob. 19.35PCh. 19 - Claisen condensation between diethyl phthalate and...Ch. 19 - Prob. 19.37PCh. 19 - Prob. 19.38PCh. 19 - Prob. 19.39PCh. 19 - Enamines normally react with methyl iodide to give...Ch. 19 - Prob. 19.41PCh. 19 - Prob. 19.42PCh. 19 - Prob. 19.43PCh. 19 - Prob. 19.44PCh. 19 - Prob. 19.45PCh. 19 - Prob. 19.46PCh. 19 - Prob. 19.47PCh. 19 - Prob. 19.48PCh. 19 - Prob. 19.49PCh. 19 - Prob. 19.50PCh. 19 - Prob. 19.51PCh. 19 - Prob. 19.52PCh. 19 - Show experimental conditions by which to carry out...Ch. 19 - Prob. 19.55PCh. 19 - The compound 3,5,5-trimethyl-2-cyclohexenone can...Ch. 19 - Prob. 19.57PCh. 19 - Prob. 19.58PCh. 19 - The widely used anticoagulant warfarin (see...Ch. 19 - Following is a retrosynthetic analysis for an...Ch. 19 - Following are the steps in one of the several...Ch. 19 - Prob. 19.62PCh. 19 - Prob. 19.63PCh. 19 - Prob. 19.65PCh. 19 - Prob. 19.67PCh. 19 - Prob. 19.68PCh. 19 - Prob. 19.69PCh. 19 - In Problem 7.28, we saw this two-step sequence in...Ch. 19 - Using your reaction roadmaps as a guide, show how...Ch. 19 - Using your reaction roadmaps as a guide, show how...Ch. 19 - Using your reaction roadmaps as a guide, show how...Ch. 19 - Using your reaction roadmaps as a guide, show how...Ch. 19 - Using your reaction roadmaps as a guide, show how...Ch. 19 - Prob. 19.79PCh. 19 - Prob. 19.80PCh. 19 - Prob. 19.81PCh. 19 - The following molecule undergoes an intramolecular...
Knowledge Booster
Similar questions
- 4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardIII O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward
- 3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forwardWhat is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forward
- Use the reaction coordinate diagram to answer the below questions. Type your answers into the answer box for each question. (Watch your spelling) Energy A B C D Reaction coordinate E A) Is the reaction step going from D to F endothermic or exothermic? A F G B) Does point D represent a reactant, product, intermediate or transition state? A/ C) Which step (step 1 or step 2) is the rate determining step? Aarrow_forward1. Using radii from Resource section 1 (p.901) and Born-Lande equation, calculate the lattice energy for PbS, which crystallizes in the NaCl structure. Then, use the Born-Haber cycle to obtain the value of lattice energy for PbS. You will need the following data following data: AH Pb(g) = 196 kJ/mol; AHƒ PbS = −98 kJ/mol; electron affinities for S(g)→S¯(g) is -201 kJ/mol; S¯(g) (g) is 640kJ/mol. Ionization energies for Pb are listed in Resource section 2, p.903. Remember that enthalpies of formation are calculated beginning with the elements in their standard states (S8 for sulfur). The formation of S2, AHF: S2 (g) = 535 kJ/mol. Compare the two values, and explain the difference. (8 points)arrow_forwardIn the answer box, type the number of maximum stereoisomers possible for the following compound. A H H COH OH = H C Br H.C OH CHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning