
(a)
Interpretation:
The way by which alkylation or acylation of an enamine can be used to convert acetophenone into the given compound has to be shown.
Concept introduction:
Enamine:
An enamine is a compound where the lone pair of electrons in the nitrogen atom of
It is formed by the reaction of a
Hydrolysis of enamine: Under acidic conditions, enamine reacts with water to give corresponding ketone and secondary amine.
Here, the bond between nitrogen and the
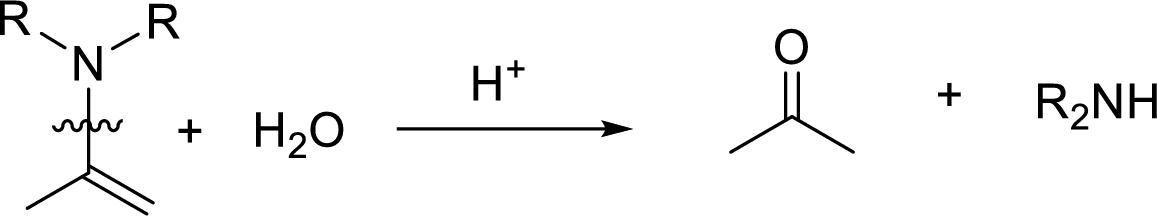
(b)
Concept introduction:
Enamine:
An enamine is a compound where the lone pair of electrons in the nitrogen atom of
It is formed by the reaction of a ketone or aldehyde and a secondary amine under acidic conditions with the elimination of water molecule.
Hydrolysis of enamine: Under acidic conditions, enamine reacts with water to give corresponding ketone and secondary amine.
Here, the bond between nitrogen and the
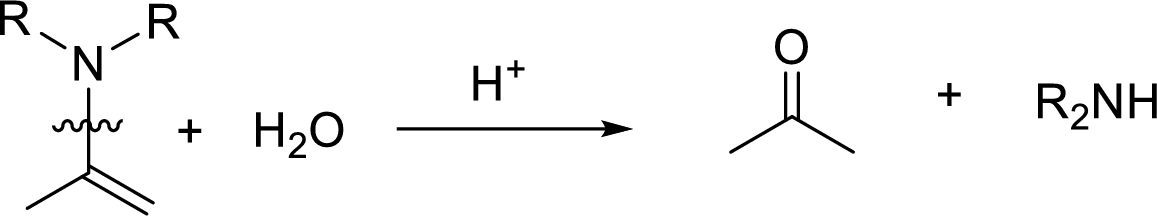
(c)
Concept introduction:
Enamine:
An enamine is a compound where the lone pair of electrons in the nitrogen atom of
It is formed by the reaction of a ketone or aldehyde and a secondary amine under acidic conditions with the elimination of water molecule.
Hydrolysis of enamine: Under acidic conditions, enamine reacts with water to give corresponding ketone and secondary amine.
Here, the bond between nitrogen and the
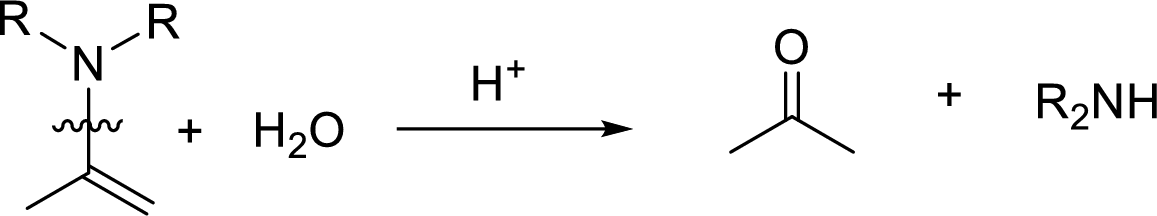
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Organic Chemistry, Loose-leaf Version
- NH2 1. CH3–MgCl 2. H3O+ ? As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C - C bond as its major product: If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C - C bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new C - C bond. Х ☐: Carrow_forwardPredict the major products of this organic reaction. If there will be no major products, check the box under the drawing area instead. No reaction. : + Х è OH K Cr O 2 27 2 4' 2 Click and drag to start drawing a structure.arrow_forwardLaminar compounds are characterized by havinga) a high value of the internal surface of the solid.b) a high adsorption potential.arrow_forward
- Intercalation compounds have their sheetsa) negatively charged.b) positively charged.arrow_forwardIndicate whether the following two statements are correct or not:- Polythiazine, formed by N and S, does not conduct electricity- Carbon can have a specific surface area of 3000 m2/garrow_forwardIndicate whether the following two statements are correct or not:- The S8 heterocycle is the origin of a family of compounds- Most of the elements that give rise to stable heterocycles belong to group d.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

