
(a)
Interpretation:
The product of the given reaction is to be predicted, considering one molar equivalent of
Concept introduction:
The C=C functional group of an alkene or the
Answer to Problem 19.54P
The product of the given reaction is
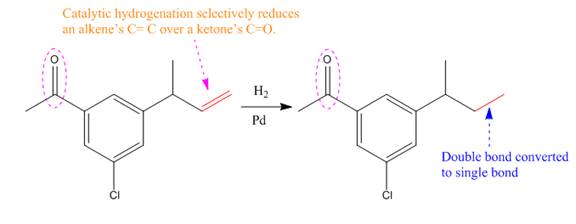
Explanation of Solution
The given reaction is
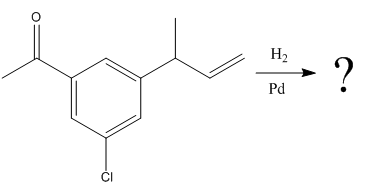
The given reaction condition correlates to catalytic hydrogenation; it reduces alkene’s C=C over ketone’s C=O. Thus, the
Therefore, the product of the given reaction is
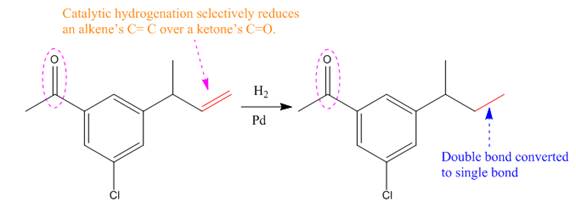
The product of the given reaction is predicted using the given reaction conditions.
(b)
Interpretation:
The product of the given reaction is to be predicted, considering one molar equivalent of
Concept introduction:
The C=C functional group of an alkene or the
Catalytic hydrogenation is more favored at a less sterically hindered multiple bond than at a more sterically hindered one.
Answer to Problem 19.54P
The product of the given reaction is
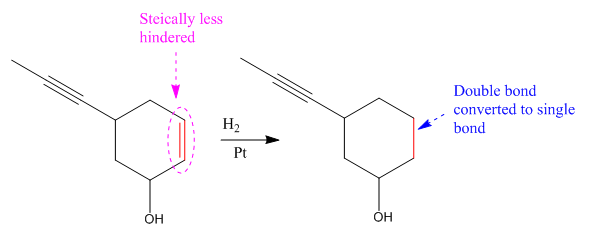
Explanation of Solution
The given reaction
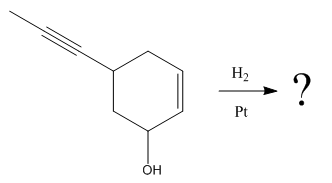
The given reaction condition correlates to catalytic hydrogenation. The substrate in the given reaction have two sites for catalytic hydrogenation, one C=C bond and another
Catalytic hydrogenation is more favored at a less sterically hindered multiple bond than at a more sterically hindered one.
In the given reaction, the C=C bond is sterically less hindered; therefore the

The product of the given reaction is predicted using given reaction conditions.
(c)
Interpretation:
The product of the given reaction is to be predicted, considering one molar equivalent of
Concept introduction:
The C=C functional group of an alkene or the
Answer to Problem 19.54P
The product of the given reaction is
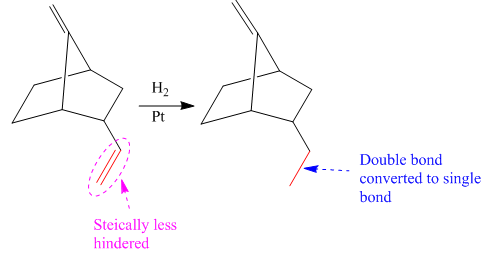
Explanation of Solution
The given reaction is
The given reaction condition correlates to catalytic hydrogenation. The substrate in the given reaction have two sites for catalytic hydrogenation, two C=C bonds.
Catalytic hydrogenation is more favored at a less sterically hindered multiple bond than at a more sterically hindered one.
In the given reaction, the C=C bond is at bottom is sterically less hindered; therefore the

The product of the given reaction is predicted using the given reaction conditions.
(d)
Interpretation:
The product of the given reaction is to be predicted, considering one molar equivalent of
Concept introduction:
The C=C functional group of an alkene or the
Answer to Problem 19.54P
The product of the given reaction is
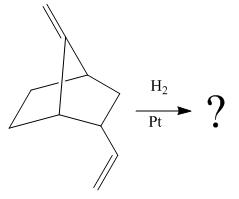
Explanation of Solution
The given reaction is
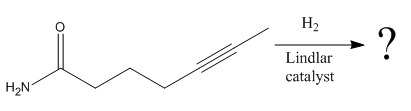
The given reaction condition correlates to catalytic hydrogenation. The substrate in the given reaction has two sites for catalytic hydrogenation, one
The functional groups in alkenes, alkynes, and aldehydes can be selectively reduced over those in ketones, nitriles, and amides. Thus, the
Therefore, the product of the given reaction is

The product of the given reaction is predicted using the given reaction conditions.
(e)
Interpretation:
The product of the given reaction is to be predicted, considering one molar equivalent of
Concept introduction:
The C=C functional group of an alkene or the
Answer to Problem 19.54P
The product of the given reaction is
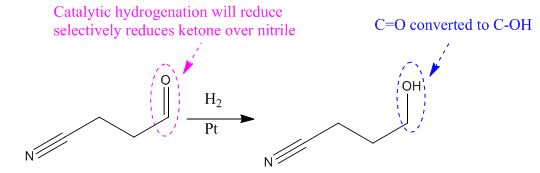
Explanation of Solution
The given reaction is
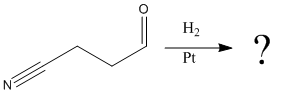
The given reaction condition correlates to catalytic hydrogenation. The substrate in the given reaction has two sites for catalytic hydrogenation, one C=C bond and another the nitrile group.
The functional group ketones can be selectively reduced over nitriles. Thus, the
Therefore, the product of the given reaction is

The product of the given reaction is predicted using the given reaction conditions.
(f)
Interpretation:
The product of the given reaction is to be predicted, considering one molar equivalent of
Concept introduction:
The C=C functional group of an alkene or the
Answer to Problem 19.54P
The product of the given reaction is
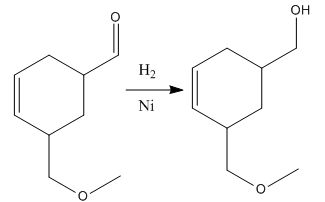
Explanation of Solution
The given reaction is
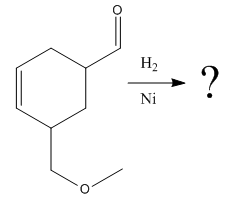
The given reaction condition correlates to catalytic hydrogenation. The substrate in the given reaction has two sites for catalytic hydrogenation, one C=C bond and another the aldehyde group.
Aldehydes and alkenes have similar reactivity toward catalytic hydrogenation, but the aldehyde group in the above substrate is less sterically hindered; thus
Therefore, the product of the given reaction is
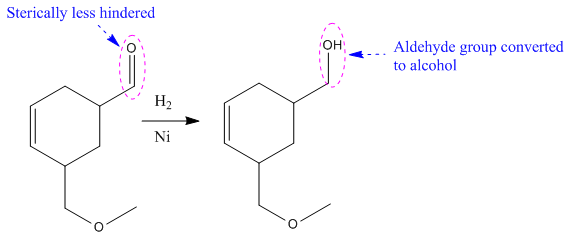
The product of the given reaction is predicted using the given reaction conditions.
(g)
Interpretation:
The product of the given reaction is to be predicted, considering one molar equivalent of
Concept introduction:
The C=C functional group of an alkene or the
Answer to Problem 19.54P
The product of the given reaction is
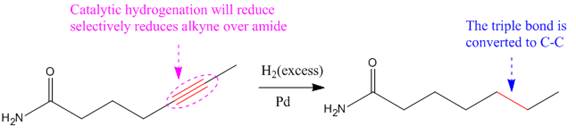
Explanation of Solution
The given reaction is
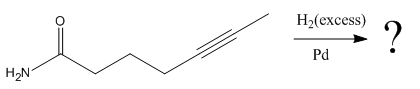
The given reaction condition correlates to catalytic hydrogenation. The substrate in the given reaction has two sites for catalytic hydrogenation, one
The functional groups in alkenes, alkynes, and aldehydes can be selectively reduced over those in ketones, nitriles, and amides. Thus, the
Therefore, the product of the given reaction is

The product of the given reaction is predicted using the given reaction conditions.
Want to see more full solutions like this?
Chapter 19 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Nonearrow_forwardIn the phase diagram of steel (two components Fe and C), region A is the gamma austenite solid and region B contains the gamma solid and liquid. Indicate the degrees of freedom that the fields A and B have,arrow_forwardFor a condensed binary system in equilibrium at constant pressure, indicate the maximum number of phases that can exist.arrow_forward
- Part V. Label ad match the carbons in compounds Jane and Diane w/ the corresponding peak no. in the Spectra (Note: use the given peak no. To label the carbons, other peak no are intentionally omitted) 7 4 2 -0.13 -0.12 -0.11 -0.10 -0.08 8 CI Jane 1 -0.09 5 210 200 190 180 170 160 150 140 130 120 110 100 -8 90 f1 (ppm) 11 8 172.4 172.0 f1 (ppr HO CI NH Diane 7 3 11 80 80 -80 -R 70 60 60 2 5 -8 50 40 8. 170 160 150 140 130 120 110 100 90 -0 80 70 20 f1 (ppm) 15 30 -20 20 -60 60 -0.07 -0.06 -0.05 -0.04 -0.03 -0.02 -0.01 -0.00 -0.01 10 -0.17 16 15 56 16 -0.16 -0.15 -0.14 -0.13 -0.12 -0.11 -0.10 -0.09 -0.08 -0.07 -0.06 -0.05 -0.04 17.8 17.6 17.4 17.2 17.0 f1 (ppm) -0.03 -0.02 550 106 40 30 20 20 -0.01 -0.00 F-0.01 10 0arrow_forwardConsider the reaction of 2-methylpropane with a halogen. With which halogen will the product be almost exclusively 2-halo-2-methylpropane? 1. F2 2. Cl2 3. Br2 4. I2arrow_forwardNonearrow_forward
- Nonearrow_forwardn Feb 3 A T + 4. (2 pts) Draw the structure of the major component of the Limonene isolated. Explain how you confirmed the structure. 5. (2 pts) Draw the fragment corresponding to the base peak in the Mass spectrum of Limonene. 6. (1 pts) Predict the 1H NMR spectral data of R-Limonene. Proton NMR: 5.3 pon multiplet (H Ringarrow_forwardPart VI. Ca H 10 O is the molecular formula of compound Tom and gives the in the table below. Give a possible structure for compound Tom. 13C Signals summarized C1 C2 C3 C4 C5 C6 C7 13C shift (ppm) 23.5 27.0 33.0 35.8 127 162 205 DEPT-90 + DEPT-135 + +arrow_forward
- 2. Using the following data to calculate the value of AvapH o of water at 298K. AvapH o of water at 373K is 40.7 kJ/mol; molar heat capacity of liquid water at constant pressure is 75.2J mol-1 K-1 and molar heat capacity of water vapor at constant pressure is 33.6 J mol-1 K-1.arrow_forwardPart VII. Below are the 'HNMR 13 3 C-NMR, COSY 2D- NMR, and HSQC 20-NMR (Similar with HETCOR but axes are reversed) spectra of an organic compound with molecular formula C6H13 O. Assign chemical shift values to the H and c atoms of the compound. Find the structure. Show complete solutions. Predicted 1H NMR Spectrum ли 4.7 4.6 4.5 4.4 4.3 4.2 4.1 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 f1 (ppm)arrow_forward3. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-pentene. expanded structure: Condensed structure: Skeletal formula: 4. Draw the expanded structural formula, the condensed structural formula, and the skeletal structural formula for 2-methyl-3-heptene. expanded structure: Condensed structure: Skeletal formula: following structurearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





