
Concept explainers
Using the
a. 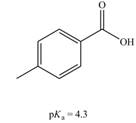 b.
b. 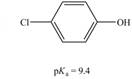 c.
c. 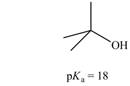
(a)
Interpretation: The correct
Concept introduction: Carboxylic acids are the carbon compounds that contain carboxyl group as a major functional group. They are polar due to electronegativity difference between the atoms in a compound. They sometimes exist as a dimer. Dimers are the compounds that consist of two monomer units connected by bonds or forces. Carboxylic acids are synthesized from alkynes, alkene, benzene derivatives, alcohol and allylic halides by using different reagents.
Answer to Problem 19.35P
The bases that are strong enough to deprotonate the given compounds are
Explanation of Solution
The given carboxylic acid is
The
Therefore, the bases that are strong enough to deprotonate the given compounds are
The bases that are strong enough to deprotonate the given compounds are
(b)
Interpretation: The correct
Concept introduction: Carboxylic acids are the carbon compounds that contain carboxyl group as a major functional group. They are polar due to electronegativity difference between the atoms in a compound. They sometimes exist as a dimer. Dimers are the compounds that consist of two monomer units connected by bonds or forces. Carboxylic acids are synthesized from alkynes, alkene, benzene derivatives, alcohol and allylic halides by using different reagents.
Answer to Problem 19.35P
The bases that are strong enough to deprotonate the given compounds are
Explanation of Solution
The given compound is
The
Therefore, the bases that are strong enough to deprotonate the given compounds are
The bases that are strong enough to deprotonate the given compounds are
(c)
Interpretation: The correct
Concept introduction: Carboxylic acids are the carbon compounds that contain carboxyl group as a major functional group. They are polar due to electronegativity difference between the atoms in a compound. They sometimes exist as a dimer. Dimers are the compounds that consist of two monomer units connected by bonds or forces. Carboxylic acids are synthesized from alkynes, alkene, benzene derivatives, alcohol and allylic halides by using different reagents.
Answer to Problem 19.35P
The bases that are strong enough to deprotonate the given compounds are
Explanation of Solution
The given carboxylic acid is
The
Therefore, the bases that are strong enough to deprotonate the given compounds are
The bases that are strong enough to deprotonate the given compounds are
Want to see more full solutions like this?
Chapter 19 Solutions
ORGANIC CHEMISTRY