
a)
Interpretation:
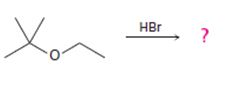
The products formed and the mechanism by which they are formed when tert-butyl ethyl ether is treated with HBr is to be given.
Concept introduction:
Ethers are cleaved by strong acids. The cleavage takes place either by SN1 or SN2 mechanisms, depending upon the structure of the substrate. Ethers with only primary and secondary alkyl groups react by SN2 mechanism. The Br- or I- attacks the protonated ether at the less hindered side to yield a single alcohol and a single
To give:
The products formed and the mechanism by which they are formed when tert-butyl ethyl ether is treated with HBr is to be given.
Answer to Problem 24MP
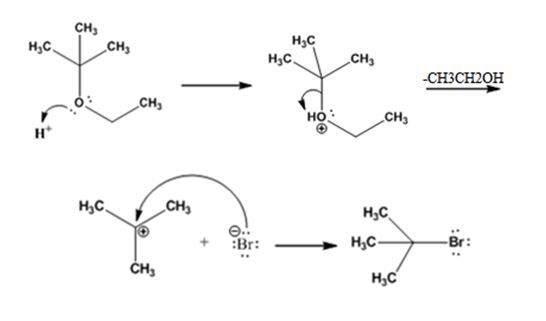
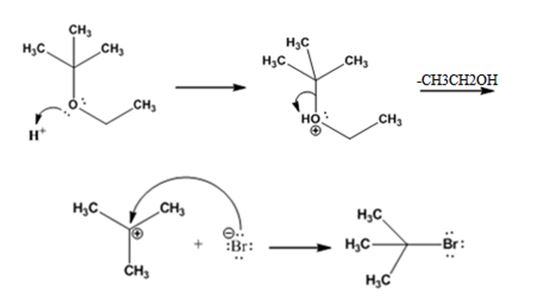
The products formed when tert-butyl ethyl ether is treated with HBr are ethanol and tert-butylbromide.
The mechanism by which they are formed is given below.
Explanation of Solution
The reaction occurs following SN1 mechanism. The acid protonates the ether initially and the protonated ether eliminates ethanol to produce a stable tert-butyl carbocation. In the next step the bromide ion attacks the carbocation to yield tert-butyl bromide as the product.
The products formed when tert-butyl ethyl ether is treated with HBr are ethanol and tert-butylbromide.
The mechanism by which they are formed is given below.
b)
Interpretation:
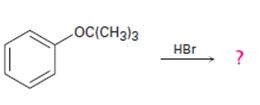
The products formed and the mechanism by which they are formed when tert-butyl phenyl ether is treated with HBr is to be given.
Concept introduction:
Ethers are cleaved by strong acids. The cleavage takes place either by SN1 or SN2 mechanisms, depending upon the structure of the substrate. Ethers with only primary and secondary alkyl groups react by SN2 mechanism. The Br- or I- attacks the protonated ether at the less hindered side to yield a single alcohol and a single alkyl halide. Ethers with a tertiary, benzylic or an allylic group cleave either by SN1 or E1 mechanism because these can produce a stable carbocations yielding alkenes and alcohols.
To give:
The products formed and the mechanism by which they are formed when tert-butyl phenyl ether is treated with HBr.
Answer to Problem 24MP
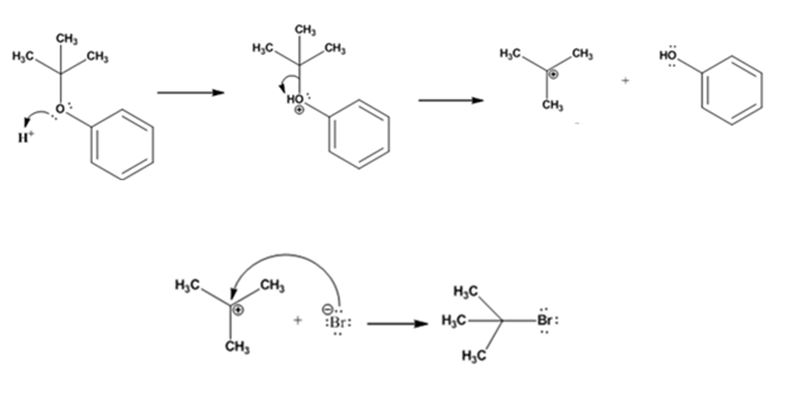
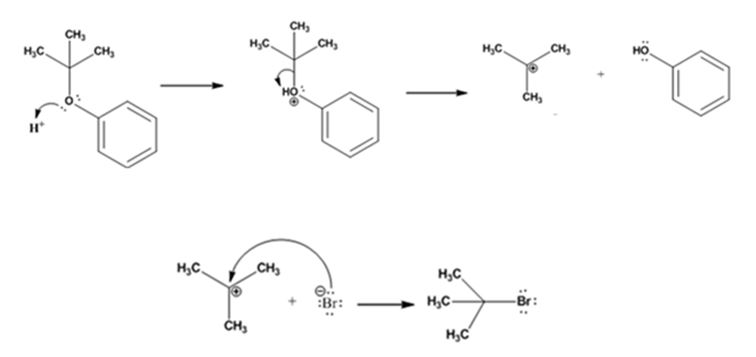
The products formed when tert-butyl phenyl ether is treated with HBr are phenol and tert-butyl bromide.
The mechanism by which they are formed is given below.
Explanation of Solution
The reaction occurs following SN1 mechanism. The acid protonates the ether initially and the protonated ether eliminates phenol to produce a stable tert-butyl carbocation. In the next step the bromide ion attacks the carbocation to yield tert-butyl bromide.
The products formed when tert-butyl phenyl ether is treated with HBr are phenol and tert-butyl bromide.
The mechanism by which they are formed is given below.
c)
Interpretation:
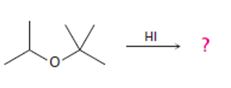
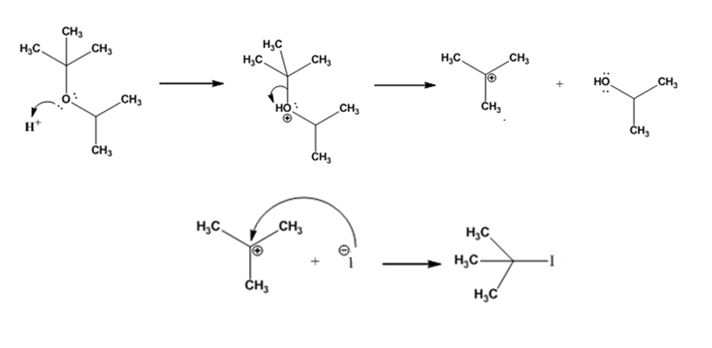
The products formed and the mechanism by which they are formed when tert-butyl isopropyl ether is treated with HI is to be given.
Concept introduction:
Ethers are cleaved by strong acids. The cleavage takes place either by SN1 or SN2 mechanisms, depending upon the structure of the substrate. Ethers with only primary and secondary alkyl groups react by SN2 mechanism. The Br- or I- attacks the protonated ether at the less hindered side to yield a single alcohol and a single alkyl halide. Ethers with a tertiary, benzylic or an allylic group cleave either by SN1 or E1 mechanism because these can produce a stable carbocations yielding alkenes and alcohols.
To give:
The products formed and the mechanism by which they are formed when tert-butyl isopropyl ether is treated with HI.
Answer to Problem 24MP
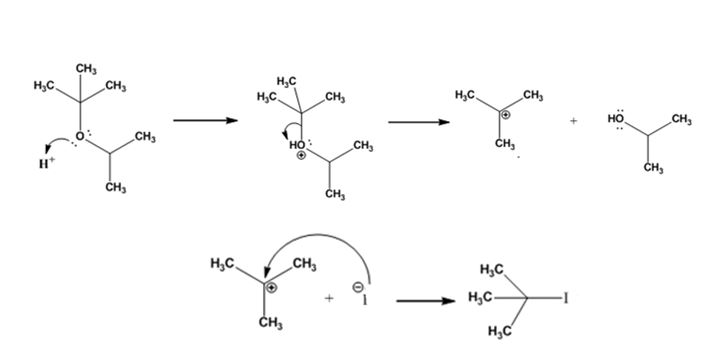
The products formed when tert-butyl isopropyl ether is treated with HI are tert-butyl bromide and 2-propanol.
The mechanism by which they are formed is given below.
Explanation of Solution
The reaction occurs following SN1 mechanism. The acid protonates the ether initially and the protonated ether eliminates 2-propanol to produce a stable tert-butyl carbocation. In the next step the iodide ion attacks the carbocation to yield tert-butyl iodide.
The products formed when tert-butyl isopropyl ether is treated with HI are tert-butyl bromide and 2-propanol.
The mechanism by which they are formed is given below.
d)
Interpretation:
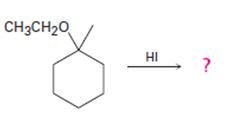
The products formed and the mechanism by which they are formed when ethyl 1-methylcyclohexyl ether is treated with HI is to be given.
Concept introduction:
Ethers are cleaved by strong acids. The cleavage takes place either by SN1 or SN2 mechanisms, depending upon the structure of the substrate. Ethers with only primary and secondary alkyl groups react by SN2 mechanism. The Br- or I- attacks the protonated ether at the less hindered side to yield a single alcohol and a single alkyl halide. Ethers with a tertiary, benzylic or an allylic group cleave either by SN1 or E1 mechanism because these can produce a stable carbocations yielding alkenes and alcohols.
To give:
The products formed and the mechanism by which they are formed when ethyl 1-methylcyclohexyl ether is treated with HI.
Answer to Problem 24MP
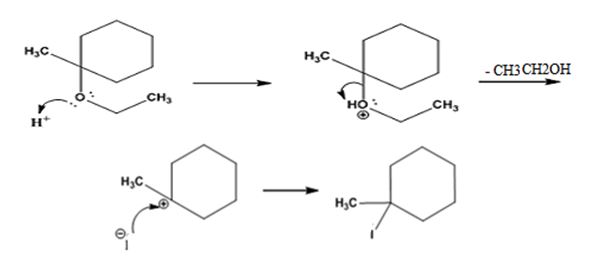
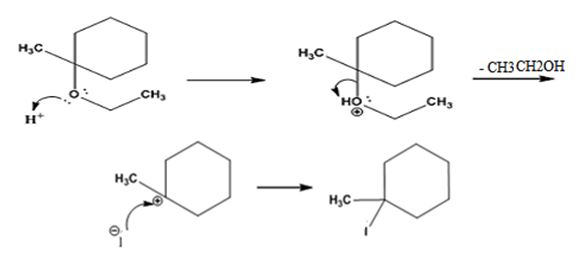
The products formed when ethyl 1-methylcyclohexyl ether is treated with HI are 1-iodo-1-methylcyclohexane and ethanol.
The mechanism by which they are formed is given below.
Explanation of Solution
The reaction occurs following SN1 mechanism. The acid protonates the ether initially and the protonated ether eliminates ethanol to produce a stable tert-butyl carbocation. In the next step the iodide ion attacks the carbocation to yield tert-butyl iodide.
The products formed when ethyl 1-methylcyclohexyl ether is treated with HI are 1-iodo-1-methylcyclohexane and ethanol.
The mechanism by which they are formed is given below.
All the reactions (a), (b), (c) and (d) occur through SN1 mechanism.
All the reactions, (a), (b), (c) and (d) take place following SN1 mechanism. The protonated
Want to see more full solutions like this?
Chapter 18 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- Assign the functional group bands on the IR spectra.arrow_forwardFind the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forward
- Draw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forwardTartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forward
- Order the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

