
Conceptual Physical Science (6th Edition)
6th Edition
ISBN: 9780134060491
Author: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 58E
Some molecules are able to stabilize a negative charge by passing it from one atom to the next by a flip-flopping of double bonds. This occurs when the negative charge is one atom away from an oxygen double bond as shown below. Note that the curved arrows indicate the movement of electrons.
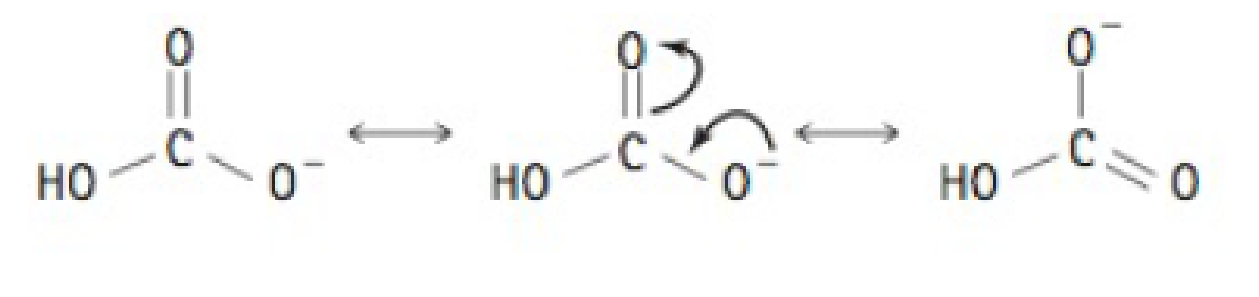
Why, then, is sulfuric acid so much stronger an acid than carbonic acid?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For each part make sure to include sign to represent direction, with up being positive and down being negative.
A ball is thrown vertically upward with a speed of 30.5 m/s.
A) How high does it rise? y=
B) How long does it take to reach its highest point? t=
C) How long does it take the ball return to its starting point after it reaches its highest point? t=
D) What is its velocity when it returns to the level from which it started? v=
Four point charges of equal magnitude Q = 55 nC are placed on the corners of a rectangle of sides D1 = 27 cm and D2 = 11cm. The charges on the left side of the rectangle are positive while the charges on the right side of the rectangle are negative. Use a coordinate system where the positive y-direction is up and the positive x-direction is to the right.
A. Which of the following represents a free-body diagram for the charge on the lower left hand corner of the rectangle?
B. Calculate the horizontal component of the net force, in newtons, on the charge which lies at the lower left corner of the rectangle.Numeric : A numeric value is expected and not an expression.Fx = __________________________________________NC. Calculate the vertical component of the net force, in newtons, on the charge which lies at the lower left corner of the rectangle.Numeric : A numeric value is expected and not an expression.Fy = __________________________________________ND. Calculate the magnitude of the…
Point charges q1=50.0μC and q2=-35μC are placed d1=1.0m apart, as shown.
A. A third charge, q3=25μC, is positioned somewhere along the line that passes through the first two charges, and the net force on q3 is zero. Which statement best describes the position of this third charge?1) Charge q3 is to the right of charge q2. 2) Charge q3 is between charges q1 and q2. 3) Charge q3 is to the left of charge q1. B. What is the distance, in meters, between charges q1 and q3? (Your response to the previous step may be used to simplify your solution.)Give numeric value.d2 = __________________________________________mC. Select option that correctly describes the change in the net force on charge q3 if the magnitude of its charge is increased.1) The magnitude of the net force on charge q3 would still be zero. 2) The effect depends upon the numeric value of charge q3. 3) The net force on charge q3 would be towards q2. 4) The net force on charge q3 would be towards q1. D. Select option that…
Chapter 18 Solutions
Conceptual Physical Science (6th Edition)
Ch. 18 - What does sulfur dioxide have to do with acid...Ch. 18 - How do humans generate the air pollutant sulfur...Ch. 18 - Prob. 14RCQCh. 18 - Prob. 15RCQCh. 18 - What elements have the greatest tendency to behave...Ch. 18 - Prob. 17RCQCh. 18 - What happens to a reducing agent as it reduces?Ch. 18 - What is electrochemistry?Ch. 18 - What is the purpose of the salt bridge in a...Ch. 18 - Prob. 21RCQ
Ch. 18 - What is the prime difference between a battery and...Ch. 18 - Prob. 23RCQCh. 18 - What is electrolysis, and how does it differ from...Ch. 18 - Prob. 25RCQCh. 18 - Prob. 26RCQCh. 18 - What metal coats a galvanized nail?Ch. 18 - Prob. 28RCQCh. 18 - What is iron forced to accept during cathodic...Ch. 18 - What happens to the polarity of oxygen atoms as...Ch. 18 - Show that the hydroxide ion concentration in an...Ch. 18 - When the hydronium ion concentration of a solution...Ch. 18 - Show that an aqueous solution having a pH of 5 has...Ch. 18 - When the pH of a solution is 1, the concentration...Ch. 18 - Show that the pH of a solution is 0.301 when its...Ch. 18 - Each year about 1.6 107 (16 million) metric tons...Ch. 18 - Prob. 44TASCh. 18 - Prob. 45TARCh. 18 - The three chemicals listed below are all very weak...Ch. 18 - Rank in order of decreasing pH the rain that fell...Ch. 18 - Prob. 48TARCh. 18 - Review the concept of electronegativity in Section...Ch. 18 - Rank the following molecules from least oxidized...Ch. 18 - An acid and a base react to form a salt, which...Ch. 18 - Identify the acid or base behavior of each...Ch. 18 - Prob. 53ECh. 18 - Prob. 54ECh. 18 - The main component of bleach is sodium...Ch. 18 - Prob. 56ECh. 18 - Prob. 57ECh. 18 - Some molecules are able to stabilize a negative...Ch. 18 - Prob. 59ECh. 18 - Within a neutral solution of supercritical water...Ch. 18 - What is the concentration of hydronium ions in a...Ch. 18 - Can an acidic solution be made less acidic by...Ch. 18 - Bubbling carbon dioxide into water causes the pH...Ch. 18 - Pour vinegar onto beach sand from the Caribbean,...Ch. 18 - What happens to the pH of soda water as it loses...Ch. 18 - Prob. 66ECh. 18 - Prob. 67ECh. 18 - Prob. 68ECh. 18 - Prob. 69ECh. 18 - Hydrogen sulfide, H2S, burns in the presence of...Ch. 18 - Unsaturated fatty acids, such as C12H22O2, react...Ch. 18 - The type of iron that the human body needs for...Ch. 18 - Chemical equations need to be balanced not only in...Ch. 18 - Prob. 74ECh. 18 - Why does a salt bridge last only so long?Ch. 18 - How does turning on the radio while you are...Ch. 18 - What are some key advantages that a fuel-cell...Ch. 18 - Why would a miniaturized fuel cell require a...Ch. 18 - Prob. 79ECh. 18 - Prob. 80ECh. 18 - Copper atoms have a greater tendency to be reduced...Ch. 18 - Clorox is a laundry bleaching agent used to remove...Ch. 18 - Pennies manufactured after 1982 are made of zinc...Ch. 18 - Prob. 84ECh. 18 - Prob. 85ECh. 18 - Water is 88.88% oxygen by mass. Oxygen is exactly...Ch. 18 - Why is the air over an open flame always moist?Ch. 18 - Upon ingestion, grain alcohol, C2H6O, is...Ch. 18 - Your body creates chemical energy from the food...Ch. 18 - Do the digestion and subsequent metabolism of...Ch. 18 - Why is it easier for the body to excrete a polar...Ch. 18 - What is the relationship between the hydroxide ion...Ch. 18 - Prob. 2RATCh. 18 - Sodium hydroxide, NaOH, is a strong base, which...Ch. 18 - Prob. 4RATCh. 18 - Why do we use the pH scale to indicate the acidity...Ch. 18 - When the hydronium ion concentration equals 1...Ch. 18 - Prob. 7RATCh. 18 - Prob. 8RATCh. 18 - How does an atum's electronegativity relate to its...Ch. 18 - Why does a battery that has thick zinc walls last...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
Cosmic Perspective Fundamentals
Name the components (including muscles) of the thoracic cage. List the contents of the thorax.
Human Physiology: An Integrated Approach (8th Edition)
How can the freezing of water crack boulders?
Campbell Biology in Focus (2nd Edition)
In cats, tortoiseshell coat color appears in females. A tortoiseshell coat has patches of dark brown fur and pa...
Genetic Analysis: An Integrated Approach (3rd Edition)
47. A block hangs in equilibrium from a vertical spring. When a second identical block is added, the original ...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
1. Which parts of the skeleton belong to the appendicular skeleton? Which belong to the axial skeleton?
Human Anatomy & Physiology (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The magnitude of the force between a pair of point charges is proportional to the product of the magnitudes of their charges and inversely proportional to the square of their separation distance. Four distinct charge-pair arrangements are presented. All charges are multiples of a common positive charge, q. All charge separations are multiples of a common length, L. Rank the four arrangements from smallest to greatest magnitude of the electric force.arrow_forwardA number of electric charges has been placed at distinct points along a line with separations as indicated. Two charges share a common magnitude, q (lower case), and another charge has magnitude Q (upper case). The signs of the charges are indicated explicitly such that ∣∣+q∣∣∣∣+Q∣∣=∣∣−q∣∣==∣∣−Q∣∣=qQ Four different configurations of charges are shown. For each, express the net electric force on the charge with magnitude Q (upper case) as F⃗E=FE,xî where the positive x direction is towards the right. By repositioning the figures to the area on the right, rank the configurations from the most negative value to the most positive value of FE,x.arrow_forwardA collection of electric charges that share a common magnitude q (lower case) has been placed at the corners of a square, and an additional charge with magnitude Q (upper case) is located at the center of that square. The signs of the charges are indicated explicitly such that ∣∣+q∣∣∣∣+Q∣∣=∣∣−q∣∣==∣∣−Q∣∣=qQ Four unique setups of charges are displayed. By moving one of the direction drawings from near the bottom to the bucket beside each of the setups, indicate the direction of the net electric force on the charge with magnitude Q, located near the center, else indicate that the magnitude of the net electric force is zero, if appropriate.arrow_forward
- In Dark Souls 3 you can kill the Ancient Wyvern by dropping on its head from above it. Let’s say you jump off the ledge with an initial velocity of 3.86 mph and spend 1.72 s in the air before hitting the wyvern’s head. Assume the gravity is the same as that of Earth and upwards is the positive direction. Also, 1 mile = 1609 m. A) How high up is the the ledge you jumped from as measured from the wyvern’s head? B) What is your velocity when you hit the wyvern?arrow_forwardA conducting sphere is mounted on an insulating stand, and initially it is electrically neutral. A student wishes to induce a charge distribution similar to what is shown here. The student may connect the sphere to ground or leave it electrically isolated. The student may also place a charged insulated rod near to the sphere without touching it. Q. The diagrams below indicate different choices for whether or not to include a ground connection as well as the sign of the charge on and the placement of an insulating rod. Choose a diagram that would produce the desired charge distribution. (If there are multiple correct answers, you need to select only one of them.)arrow_forwardA person is making pancakes and tries to flip one in the pan. The person is holding the pan a distance y0 = 1.10 m above the ground when they launch the pancake. The pancake just barely touches the ceiling, which is at a height y = 2.47 m above the ground. A) What must be the initial velocity of the pancake to reach that height? B) This person, shocked that they almost hit the ceiling, does not catch it on the way down and the pancake hits the floor. Assuming up as the positive direction, what is the velocity of the pancake when it hits the floor, ruining breakfast and this person’s day?arrow_forward
- One of Spider-Man’s less talked about powers is that he can jump really high. In the comics Spider-Man can jump upwards 3 stories. A) If Spider-Man leaves the ground at 14.3 m/s, how high can he get? y= B) If Spider-Man jumps directly upwards with the initial velocity used above and then returns to the ground, what total amount of time does he spend airborn? t=arrow_forwardAn insulating rod is positively charged, and an electrically neutral conducting sphere is mounted on an insulating stand. The rod is brought near to the sphere on the right, but they never actually touch. Q. Select the image that best represents the resulting charge distribution on the conducting sphere.arrow_forwardThis is a multi-part problem. For each part make sure to include sign to represent direction, with up being positive and down being negative. A ball is thrown vertically upward with a speed of 30.5 m/s. A) How high does it rise? y= B) How long does it take to reach its highest point? t= C) How long does it take the ball return to its starting point after it reaches its highest point? t= D) What is its velocity when it returns to the level from which it started? v=arrow_forward
- Blue light has a wavelength of 485 nm. What is the frequency of a photon of blue light? Question 13 Question 13 What is the wavelength of radiofrequency broadcast of 104 MHz? Question 14 Question 14 1 Point 3. The output intensity from an x-ray exposure is 4 mGy at 90 cm. What will the intensity of the exposure be at 180 cm? Question 15 Question 15 1 Point What is the frequency of an 80 keV x-ray?arrow_forwardUnder what condition is IA - BI = A + B? Vectors À and B are in the same direction. Vectors À and B are in opposite directions. The magnitude of vector Vectors À and 官 B is zero. are in perpendicular directions.arrow_forwardFor the vectors shown in the figure, express vector 3 in terms of vectors M and N. M S =-M+ Ň == S=м- Ñ S = M +Ñ +Narrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY