
(a)
Interpretation:
The ammonium salt produced by the reaction between HCl and coniine alkaloid should be determined.
Concept Introduction:
Alkaloids are natural organic compounds present in plants. They are
Amines have basic properties, meaning they can act as proton acceptors.
Because of the basicity, amines react with acids such as HCl to form water-soluble ammonium salts. Below shown is the general reaction between a primary amine and HCl.
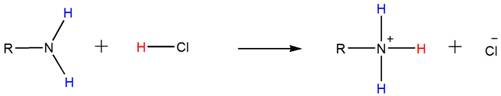
Accordingly, both the secondary and tertiary amines also form their respective ammonium salts with HCl.
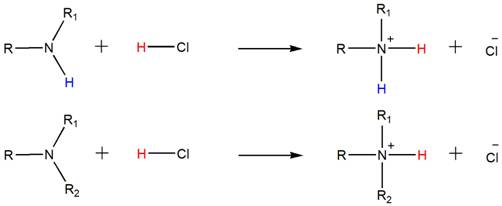
(b)
Interpretation:
The ammonium salt produced by the reaction between HCl and morphine alkaloid should be determined.
Concept Introduction:
Alkaloids are natural organic compounds present in plants. They are amines.
Amines have basic properties, meaning they can act as proton acceptors.
Because of the basicity, amines react with acids such as HCl to form water-soluble ammonium salts. Below shown is the general reaction between a primary amine and HCl.
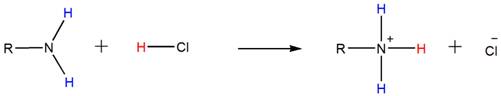
Accordingly, both the secondary and tertiary amines also form their respective ammonium salts with HCl.
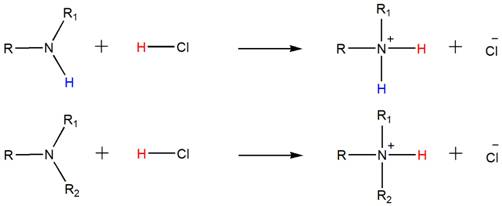
Want to see the full answer?
Check out a sample textbook solution
Chapter 18 Solutions
Connect One Semester Access Card for General, Organic, & Biological Chemistry
- Hi I need help on the question provided in the image.arrow_forwardDraw a reasonable mechanism for the following reaction:arrow_forwardDraw the mechanism for the following reaction: CH3 CH3 Et-OH Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. L CONT. י Α [1] осн CH3 а CH3 :Ö Et H 0 N о S 0 Br Et-ÖH | P LL Farrow_forward
- 20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co



