
Interpretation:
The short sequences of reactions that would be appropriate for the given transformations, using the indicated starting material are to be written.
Concept introduction:
Friedel Craft acylation is an electrophilic
Grignard reaction is a reaction in which aryl or
The molecule,
Dess Martin periodinane (DMP) is a reagent which is used for the oxidation of primary alcohols to
The Diels–Alder reaction is a
Answer to Problem 42P
Solution:
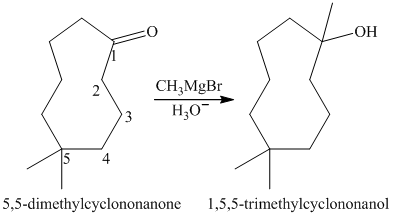
a) Short sequences of reactions that are appropriate for the transformation of
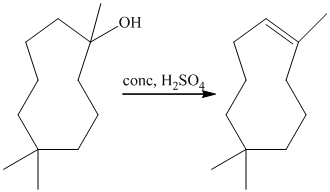
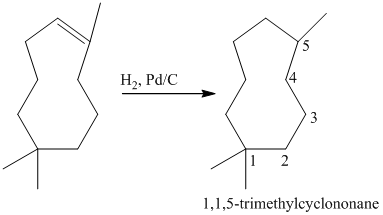
b) Short sequences of reactions for the transformation of the given compounds are shown below.
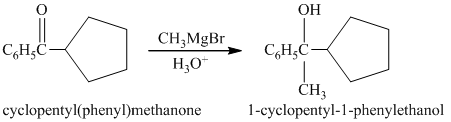
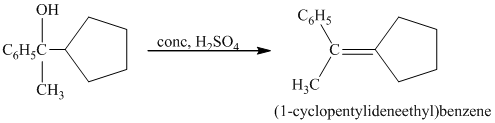
c) Short sequences of reactions for the transformation of the given compound from
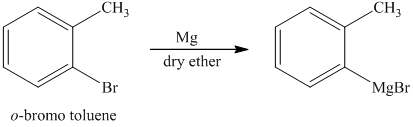
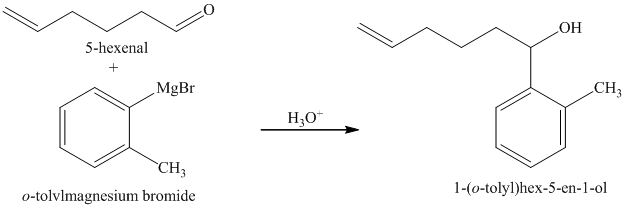
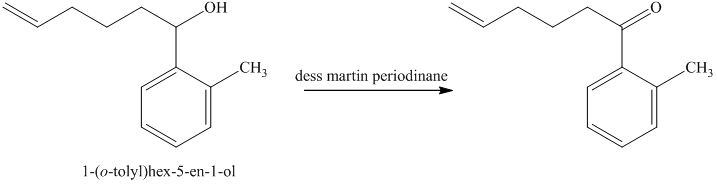
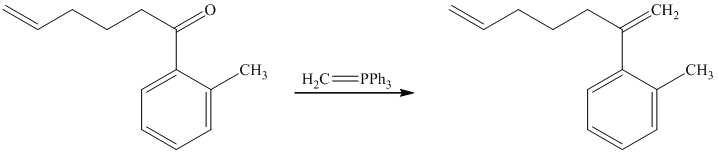
d) Short sequences of reactions for the transformation of the given compounds are shown below.

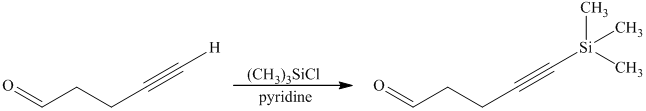
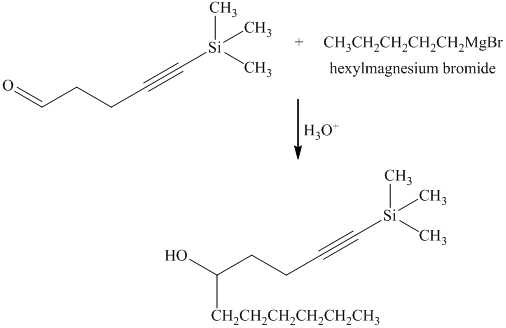
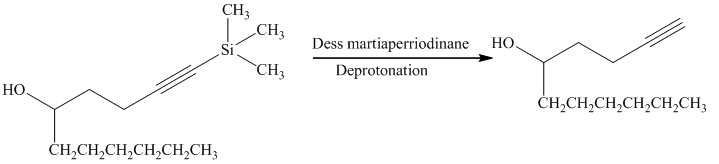
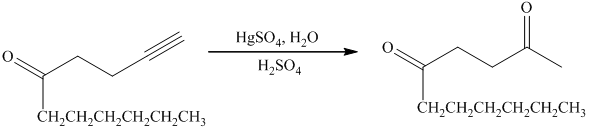
e) Short sequences of reactions that are appropriate for the transformation of the given compound from
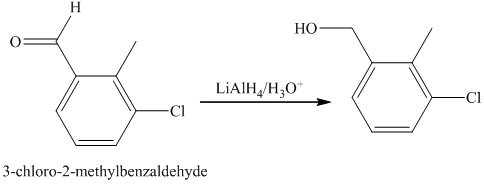
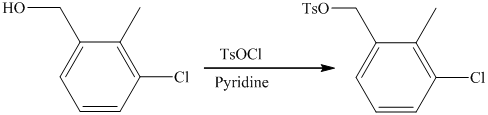
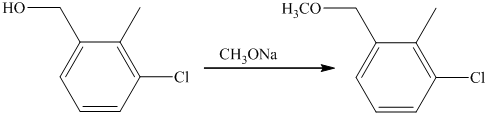
f) Short sequences of reactions that are appropriate for the transformation of the given compounds are shown below.
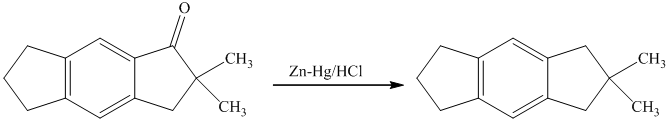
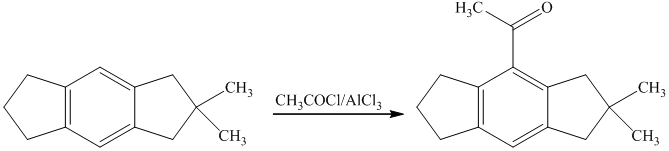
g) Short sequences of reactions that are appropriate for the transformation of the given compounds are shown below.
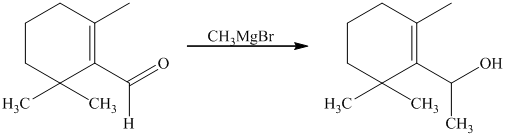
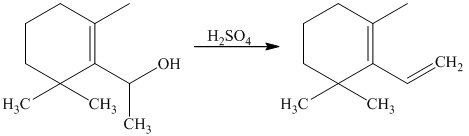
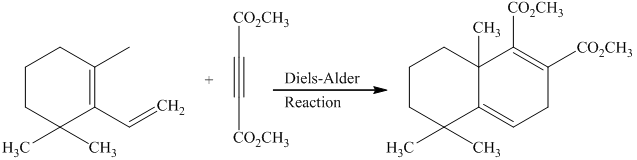
Explanation of Solution
a) Short sequences of reactions that would be appropriate for the transformation of
Treatment of
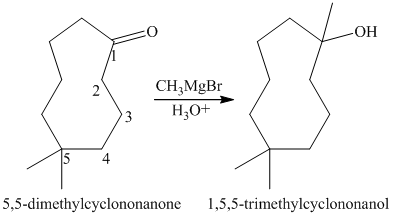
The tertiary alcohol so obtained undergoes dehydration with conc.

Reduction of the alkene with

b) Short sequences of reactions that would be appropriate for the transformation of the compounds given below have to be decribed.
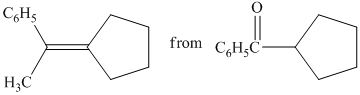
Treatment of cyclopentyl(phenyl) methanone with griginard reagent

The tertiary alcohol, upon dehydration with conc.

c) The structure of the compound which has to be synthesized from
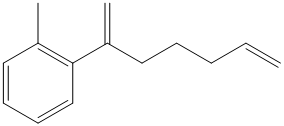
The reaction of

The griginard reagent formed reacts with

The alcohol formed reacts with dess martin periodinane to form ketone as shown in the reaction below.

The ketone reacts with Wittig reagent

d) Short sequences of reactions that would be appropriate for the transformation of the compounds given below have to be described.
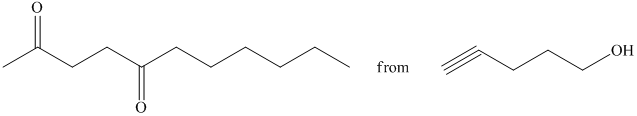
Oxidation of the given alcohol using pyridinium dichromate (PDC) in

Protection of acidic proton of is done by reaction of the aldehyde with

Reaction of the aldehyde with the griginard reagent, hexylmagnesium chloride, produces an alcohol as shown in the reaction below.

Alcohols react with martin dess periodinane to form ketone as shown below.

Terminal alkynes are converted to ketones by reaction with

e) The structure of the compound which has to be synthesized from
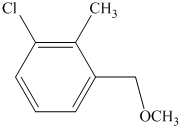
In the reduction of

The hydroxyl group of the alcohol is a poor leaving group and therefore, it is reacted with

The

f) Short sequences of reactions that would be appropriate for the transformation of the compounds given below have to be decribed.
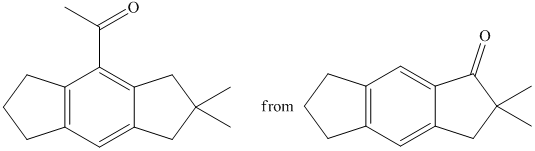
Reduction of the ketone shown above with zinc-amalgam produces a hydrocarbon in Clemmenson’s reduction as shown in the reaction below.

Friedal Crafts acylation of the aromatic ring obtained with

g) Short sequences of reactions that would be appropriate for the transformation of the compounds given below have to be decribed.
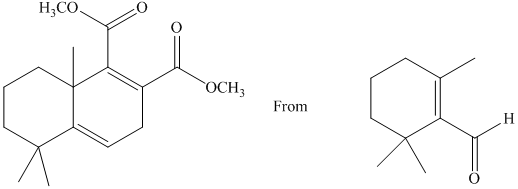
Treatment of the reactant with griginard reagent

The tertiary alcohol so obtained undergoes dehydration with conc.

Diels-Alder reaction of the diene with the dienophile produces the desired product as shown in the reaction below.

Want to see more full solutions like this?
Chapter 18 Solutions
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward

 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


