
ORGANIC CHEMISTRY W/ALEKS
6th Edition
ISBN: 9781264905430
Author: SMITH
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 40P
Give the IUPAC name for each compound.
a. 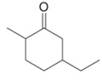 c.
c. 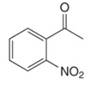 e.
e. 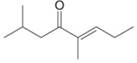
b. 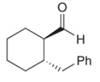 d.
d. 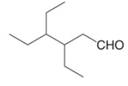 f.
f. 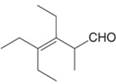
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Determine the pH of solution of HC3H5O2 By constructing an ICE table writing the equilibrium constant expression, and using this information to determine the pH. The Ka of HC3H5O2 is 1.3 x 10-5
Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction LiNO3
An unknown weak acid with a concentration of 0.410 M has a pH of 5.600. What is the Ka of the weak acid?
Chapter 18 Solutions
ORGANIC CHEMISTRY W/ALEKS
Ch. 18.1 - Rank the following compounds in order of...Ch. 18.1 - Prob. 2PCh. 18.2 - Give the IUPAC name for each aldehyde.Ch. 18.2 - Prob. 4PCh. 18.2 - Give the IUPAC name for each ketone.Ch. 18.5 - Prob. 11PCh. 18.9 - Problem 21.17 Draw the products of the following...Ch. 18.9 - Problem 21.18 Outline a synthesis of each Wittig...Ch. 18.9 - Problem 21.19 Draw the products (including...Ch. 18.9 - Problem 21.20 What starting materials are needed...
Ch. 18.9 - Prob. 19PCh. 18.10 - Problem 21.22 The product formed when reacts with...Ch. 18.10 - Prob. 21PCh. 18.11 - Prob. 22PCh. 18.11 - Prob. 23PCh. 18.11 - Prob. 24PCh. 18.12 - Prob. 25PCh. 18.12 - Problem 21.28 Draw a stepwise mechanism for the...Ch. 18.13 - Problem 21.29 Draw the products of each...Ch. 18 - Problem 21.40 (a) Give the IUPAC name for A and B....Ch. 18 - 21.41 Rank the following compounds in order of...Ch. 18 - Prob. 39PCh. 18 - 21.43 Give the IUPAC name for each compound.
a....Ch. 18 - 21.44 Give the structure corresponding to each...Ch. 18 - Prob. 42PCh. 18 - 21.46 Draw the products of each reaction.
a. e....Ch. 18 - Prob. 44PCh. 18 - 21.48 Draw all stereoisomers formed in each...Ch. 18 - Prob. 54PCh. 18 - Prob. 55PCh. 18 - Prob. 56PCh. 18 - Devise a synthesis of each alkene using a Wittig...Ch. 18 - Prob. 60PCh. 18 - Prob. 62PCh. 18 - Prob. 63PCh. 18 - 21.64 Draw a stepwise mechanism for the following...Ch. 18 - 21.65 Draw a stepwise mechanism f or the following...Ch. 18 - Prob. 67PCh. 18 - 21.67 Draw a stepwise mechanism for each...Ch. 18 - Prob. 69PCh. 18 - Prob. 70P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
Some people compare DNA to a blueprint stored in the office of a construction company. Explain how this analogy...
Biology: Concepts and Investigations
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (racemic) 19.84 Using your reaction roadmaps as a guide, show how to convert 2-oxepanone and ethanol into 1-cyclopentenecarbaldehyde. You must use 2-oxepanone as the source of all carbon atoms in the target molecule. Show all reagents and all molecules synthesized along the way. & + EtOH H 2-Oxepanone 1-Cyclopentenecarbaldehydearrow_forwardR₂ R₁ R₁ a R Rg Nu R₂ Rg R₁ R R₁₂ R3 R R Nu enolate forming R₁ R B-Alkylated carbonyl species or amines Cyclic B-Ketoester R₁₁ HOB R R₁B R R₁₂ B-Hydroxy carbonyl R diester R2 R3 R₁ RB OR R₂ 0 aB-Unsaturated carbonyl NaOR Aldol HOR reaction 1) LDA 2) R-X 3) H₂O/H₂O ketone, aldehyde 1) 2°-amine 2) acid chloride 3) H₂O'/H₂O 0 O R₁ R₁ R R₁ R₁₂ Alkylated a-carbon R₁ H.C R₁ H.C Alkylated methyl ketone acetoacetic ester B-Ketoester ester R₁ HO R₂ R B-Dicarbonyl HO Alkylated carboxylic acid malonic ester Write the reagents required to bring about each reaction next to the arrows shown. Next, record any regiochemistry or stereochemistry considerations relevant to the reaction. You should also record any key aspects of the mechanism, such as forma- tion of an important intermediate, as a helpful reminder. You may want to keep track of all reactions that make carbon-carbon bonds, because these help you build large molecules from smaller fragments. This especially applies to the reactions in…arrow_forwardProvide the reasonable steps to achieve the following synthesis.arrow_forward
- (a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forwardConsider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning  Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY