
Chemistry
4th Edition
ISBN: 9780078021527
Author: Julia Burdge
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1.8, Problem 1PPC
Practice Problem CONCEPTUALIZE
CONCEPTUALIZE
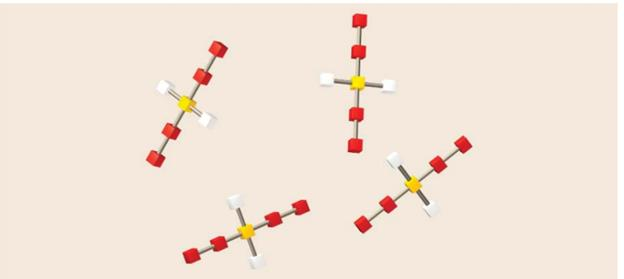
The diagram contains several objects that are constructed using colored blocks and grey connectors. Note that each of the objects is essentially identical, consisting of the same number and arrangement of blocks and connectors. Give the appropriate conversion factor for each of the specified operations.
(a)
We know the number of objects and wish to determine the number of red blocks.
(b)
We know the number of yellow blocks and wish to determine the number of objects.
(c)
We know the number of yellow blocks and wish to determine the number of white blocks.
(d)
We know the number of grey connectors and wish to determine the number of yellow blocks.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
how many moles of H2O2 are required to react with 11g of N2H4 according to the following reaction? (atomic weights: N=14.01, H=1.008, O= 16.00) 7H2O2 + N2H4 -> 2HNO3 + 8H20
calculate the number of moles of H2 produced from 0.78 moles of Ga and 1.92 moles HCL? 2Ga+6HCL->2GaCl3+3H2
an adult human breathes 0.50L of air at 1 atm with each breath. If a 50L air tank at 200 atm is available, how man y breaths will the tank provide
Chapter 1 Solutions
Chemistry
Ch. 1.1 - Practice Problem ATTEMPT
Express the freezing...Ch. 1.1 - Practice ProblemBUILD According to the website of...Ch. 1.1 - Prob. 1PPCCh. 1.2 - Prob. 1PPACh. 1.2 - Practice ProblemBUILD In Ray Bradbury's 1953 novel...Ch. 1.2 - Practice ProblemCONCEPTUALIZE If a single degree...Ch. 1.3 - Practice ProblemAttempt Given that 25 .0 mL of...Ch. 1.3 - Practice Problem BUILD
Calculate (a) the density...Ch. 1.3 - Practice ProblemCONCEPTUALIZE Using the picture of...Ch. 1.3 - The coldest temperature ever recorded on Earth was...
Ch. 1.3 - What is the density of an object that has a volume...Ch. 1.3 - A sample of water is heated from room temperature...Ch. 1.3 - Prob. 4CPCh. 1.4 - Prob. 1PPACh. 1.4 - Prob. 1PPBCh. 1.4 - Practice Problem CONCEPTUALIZE
The diagram on the...Ch. 1.4 - Prob. 1CPCh. 1.4 - Prob. 2CPCh. 1.5 - Practice Problem ATTEMPT
Determine the number of...Ch. 1.5 - Practice ProblemBUILD For each of the following...Ch. 1.5 - Prob. 1PPCCh. 1.5 - 1.5.1 What volume of water does the graduated...Ch. 1.5 - Which of the following is the sum of the following...Ch. 1.5 - The true dependence of y on x is represented by...Ch. 1.5 - What is the result of the following calculation to...Ch. 1.6 - Practice ProblemATTEMPT Perform the following...Ch. 1.6 - Practice ProblemBUILD Perform the following...Ch. 1.6 - Practice Problem CONCEPTUALIZE
A citrus dealer in...Ch. 1.6 - The density of lithium metal is 535 kg/m 3 . What...Ch. 1.6 - 1.6.2 Convert to liters.
Ch. 1.6 - 1.6.3 What is the volume of a 5.75-g object that...Ch. 1.6 - How many cubic centimeters are there in a cubic...Ch. 1.7 - Practice Problem ATTEMPT
An empty container with...Ch. 1.7 - Practice Problem BUILD
Another empty container...Ch. 1.7 - Practice ProblemCONCEPTUALIZE Several pieces of...Ch. 1.8 - Practice ProblemATTEMPT The American Heart...Ch. 1.8 - Practice ProblemBUILD An object has a mass of...Ch. 1.8 - Practice ProblemCONCEPTUALIZE The diagram contains...Ch. 1.9 - Practice ProblemATTEMPT The density of silver is...Ch. 1.9 - Practice ProblemBUILD The density of mercury is 13...Ch. 1.9 - Practice ProblemCONCEPTUALIZE Each diagram [ ( i )...Ch. 1 - 1.1
Given that the density of gold is , calculate...Ch. 1 - Prob. 2KSPCh. 1 - Determine the density of the following object in...Ch. 1 - A 28-kg child can consume a maximum of 23...Ch. 1 - Define the terms chemistry and matter.Ch. 1 - 1.2 Explain what is meant by the scientific...Ch. 1 - what is the difference between a hypothesis and a...Ch. 1 - Classify each of the following statements as a...Ch. 1 - Classify each of the following statements as a...Ch. 1 - Identify the elements present in the following...Ch. 1 - Identify the elements present in the following...Ch. 1 - Give an example for each of the following terms:...Ch. 1 - 1.9 Give an example of a homogeneous mixture and...Ch. 1 - Give an example of an element and a compound. How...Ch. 1 - What is the number of known elements?Ch. 1 - Give the names of the elements represented by the...Ch. 1 - 1.13 Give the chemical symbols for the following...Ch. 1 - Classify each of the following substances as an...Ch. 1 - Classify each of the following as an element, a...Ch. 1 - Identify each of the diagrams shown here as a...Ch. 1 - Identify each of the diagrams shown here as an...Ch. 1 - Name the SI base units that are important in...Ch. 1 - 1.19 Write the numbers represented by the...Ch. 1 - 1.20 What units do chemists normally use for the...Ch. 1 - 1.21 What is the difference between mass and...Ch. 1 - 1.22 Describe the three temperature scales used in...Ch. 1 - Bromine is a reddish-brown liquid. Calculate its...Ch. 1 - 1.24 The density of ethanol, a colorless liquid...Ch. 1 - Prob. 25QPCh. 1 - Prob. 26QPCh. 1 - 1.27 The density of water at is . What is the...Ch. 1 - The density of platinum (Pt) is 21 .5 g/cm 3 at...Ch. 1 - Convert the following temperatures to kelvin: (a)...Ch. 1 - Convert the following temperatures to degrees...Ch. 1 - 1.31 Which of the following illustrations best...Ch. 1 - The diagram shows balls of aluminum foil dropped...Ch. 1 - What is the difference between qualitative data...Ch. 1 - Using examples, explain the difference between a...Ch. 1 - How does an intensive property differ from an...Ch. 1 - Determine which of the following properties are...Ch. 1 - Classify the following as qualitative or...Ch. 1 - 1.38 Determine whether the following statements...Ch. 1 - Determine whether each of the following describes...Ch. 1 - 1.40 A student pours 44.3 g of water at into a...Ch. 1 - Prob. 41QPCh. 1 - Comment on whether each of the following...Ch. 1 - What is the advantage of using scientific notation...Ch. 1 - Define significant figure. Discuss the importance...Ch. 1 - Distinguish between the terms accuracy and...Ch. 1 - 1.46 Express the following numbers in scientific...Ch. 1 - Express the following as decimals: (a) 1.52 × 10 −...Ch. 1 - Express the answers to the following calculations...Ch. 1 - 1.49 Express the answers to the following...Ch. 1 - 1.50 Determine the number of significant figures...Ch. 1 - Determine the number of significant figures in...Ch. 1 - Prob. 52QPCh. 1 - Carry out the following operations as if they were...Ch. 1 - Three students ( A, B, and C ) are asked to...Ch. 1 - Three apprentice tailors ( X, Y, and Z ) are...Ch. 1 - Carry out the following conversions: (a) 22.6 m to...Ch. 1 - Carry out the following conversions: (a) 242 lb to...Ch. 1 - The average speed of helium at 25°C is 1255 m/s ....Ch. 1 - Prob. 59QPCh. 1 - Prob. 60QPCh. 1 - Prob. 61QPCh. 1 - 1.62 A 6.0-ft person weighs 168 lb. Express this...Ch. 1 - The highest speed limit in the United States is 85...Ch. 1 - For a fighter jet to take off from the deck of an...Ch. 1 - Prob. 65QPCh. 1 - Prob. 66QPCh. 1 - Carry out the following conversions: (a) 185 nm to...Ch. 1 - 1.68 Aluminum is a lightweight metal used in...Ch. 1 - Prob. 69QPCh. 1 - (a) Carbon monoxide ( CO ) is a poisonous gas...Ch. 1 - Prob. 71QPCh. 1 - A human brain weighs about 1 kg and contains about...Ch. 1 - Using the appropriate number of significant...Ch. 1 - 1.74 A piece of metal with a mass of 13.2 g was...Ch. 1 - 1.75 Which of the following statements describe...Ch. 1 - 1.76 In determining the density of a rectangular...Ch. 1 - Calculate the mass of each of the following: (a) a...Ch. 1 - 1.78 A cylindrical glass tube 12.7 cm in length is...Ch. 1 - The following procedure was used to determine the...Ch. 1 - 1.80 The speed of sound in air at room temperature...Ch. 1 - A piece of silver ( Ag ) metal weighing 194.3 g is...Ch. 1 - The experiment described in Problem 1.81 is a...Ch. 1 - A lead sphere has a mass of 1 .20 × 10 4 g . and...Ch. 1 - Lithium is the least dense metal known ( density =...Ch. 1 - At what temperature does the numerical reading on...Ch. 1 - Prob. 86APCh. 1 - Prob. 87APCh. 1 - A sheet of aluminum ( A1 ) foil hat a total area...Ch. 1 - Prob. 89APCh. 1 - 1.90 The surface area and average depth of the...Ch. 1 - The unit "troy ounce" is often used for precious...Ch. 1 - Prob. 92APCh. 1 - Calculate the percent error for the following...Ch. 1 - In water conservation, chemists spread a thin film...Ch. 1 - 1.95 You are given a liquid. Briefly describe the...Ch. 1 - A gas company in Massachusetts charges $1 .30 for...Ch. 1 - Prob. 97APCh. 1 - 1.98 A bank teller is asked to assemble sets of...Ch. 1 - The men's world record for running a mile outdoors...Ch. 1 - 1.100 Venus, the second closest planet to the sun....Ch. 1 - Comment on whether each of the following is a...Ch. 1 - It has been estimated that 8.0 × 10 4 tons of gold...Ch. 1 - Prob. 103APCh. 1 - 1.104 Measurements show that 1.0 g of iron ...Ch. 1 - 1.105 The thin outer layer of Earth, called the...Ch. 1 - 1.106 The radius of a copper atom is roughly ....Ch. 1 - A graduated cylinder is filled to the 40.00-mL...Ch. 1 - A chemist mixes two liquids A and B to form a...Ch. 1 - A chemist in the nineteenth century prepared an...Ch. 1 - Chlorine is used to disinfect swimming pools. The...Ch. 1 - Prob. 111APCh. 1 - Prob. 112APCh. 1 - Chalcopyrite, the principal one of copper ( Cu ) ,...Ch. 1 - 1.114 Vanillin (used to flavor vanilla ice cream...Ch. 1 - One gallon of gasoline in an automobile’s engine...Ch. 1 - 1.116 Magnesium (Mg) is a valuable metal used in...Ch. 1 - Prob. 117APCh. 1 - The natural abundances of elements in the human...Ch. 1 - A resting adult requires about 240 mL of pure...Ch. 1 - 1.120 (a) Referring to Problem 1.119. calculate...Ch. 1 - The medicinal thermometer commonly used in homes...Ch. 1 - TUMS is a popular remedy for acid indigestion. A...Ch. 1 - Prob. 123APCh. 1 - English writer and essayist Lady Mary Wortley...Ch. 1 - English writer and essayist Lady Mary Wortley...Ch. 1 - English writer and essayist Lady Mary Wortley...Ch. 1 - English writer and essayist Lady Mary Wortley...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NO2 (g) = N2O4(g) AGº = -5.4 kJ Now suppose a reaction vessel is filled with 4.53 atm of dinitrogen tetroxide (N2O4) at 279. °C. Answer the following questions about this system: Under these conditions, will the pressure of N2O4 tend to rise or fall? Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to '2' rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. 00 rise ☐ x10 fall yes no ☐ atm G Ar 1arrow_forwardWhy do we analyse salt?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H H CH3OH, H+ H Select to Add Arrows H° 0:0 'H + Q HH ■ Select to Add Arrows CH3OH, H* H. H CH3OH, H+ HH ■ Select to Add Arrows i Please select a drawing or reagent from the question areaarrow_forward
- What are examples of analytical methods that can be used to analyse salt in tomato sauce?arrow_forwardA common alkene starting material is shown below. Predict the major product for each reaction. Use a dash or wedge bond to indicate the relative stereochemistry of substituents on asymmetric centers, where applicable. Ignore any inorganic byproducts H Šali OH H OH Select to Edit Select to Draw 1. BH3-THF 1. Hg(OAc)2, H2O =U= 2. H2O2, NaOH 2. NaBH4, NaOH + Please select a drawing or reagent from the question areaarrow_forwardWhat is the MOHR titration & AOAC method? What is it and how does it work? How can it be used to quantify salt in a sample?arrow_forward
- Predict the major products of this reaction. Cl₂ hv ? Draw only the major product or products in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If there will be no products because there will be no significant reaction, just check the box under the drawing area and leave it blank. Note for advanced students: you can ignore any products of repeated addition. Explanation Check Click and drag to start drawing a structure. 80 10 m 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility DII A F1 F2 F3 F4 F5 F6 F7 F8 EO F11arrow_forwardGiven a system with an anodic overpotential, the variation of η as a function of current density- at low fields is linear.- at higher fields, it follows Tafel's law.Calculate the range of current densities for which the overpotential has the same value when calculated for both cases (the maximum relative difference will be 5%, compared to the behavior for higher fields).arrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2 (g) + 3H2 (g) = 2NH3 (g) AGº = -34. KJ Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2 tend to rise or fall? ☐ x10 fall Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of N2 will tend to rise, can that be changed to a tendency to fall by adding H2? Similarly, if you said the pressure of N will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm Х ด ? olo 18 Ararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY