
(a)
Interpretation:
The complete, detailed mechanism of a given reaction in mildly acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to
Answer to Problem 18.57P
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
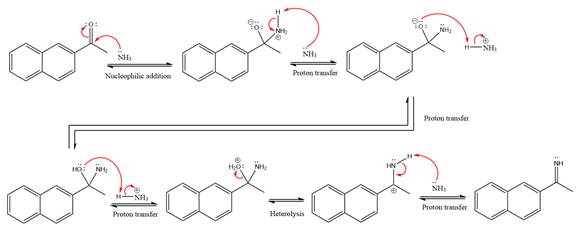
Explanation of Solution
The given reaction is
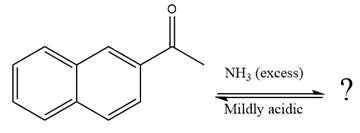
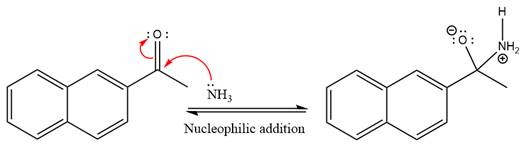
This is an imine formation reaction in which the reaction is catalyzed under mildly acidic condition and the excess ammonia acts as the nucleophile. Ammonia attacks the
First three steps are the nucleophilic addition reactions on the ketone. In the first step, nucleophile
In the next step, deprotonation takes place by ammonia
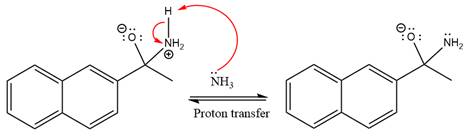
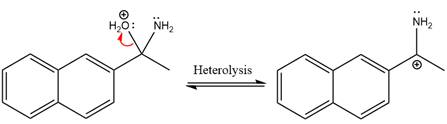
Next, negativively charged oxygen ion abstracts the proton from the ammonium ion.
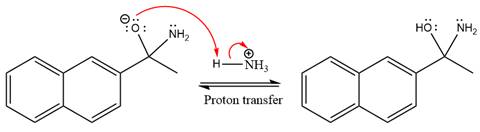
The lone pairs on
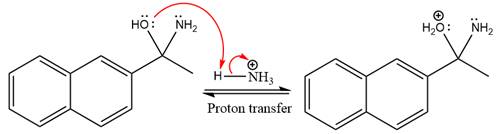
The
Ammonia nucleophile abstracts the proton from the amino group and forms imine as the product.
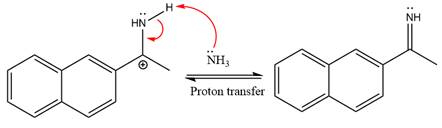
The complete, detailed mechanism of the given reaction under mildly acidic medium is shown below and an imine is the major product.
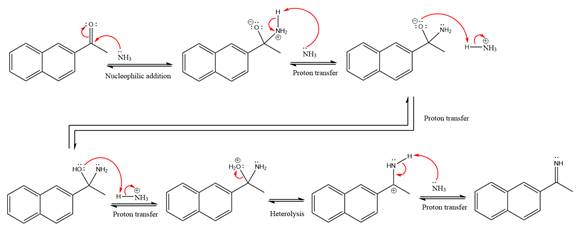
The complete, detailed mechanism of the given reaction under mildly acidic medium and excess ammonia is drawn.
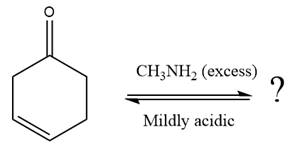
(b)
Interpretation:
The complete, detailed mechanism of a given reaction in the acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to ketone or an aldehyde, the uncharged nucleophile ammonia (
Answer to Problem 18.57P
The complete, detailed mechanism of the given reaction in the acidic medium is shown below and an acetal is the major product.
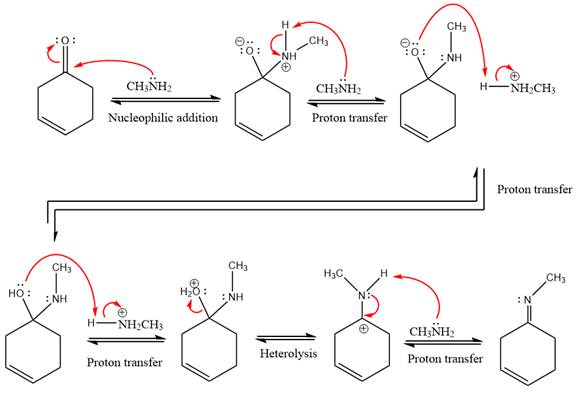
Explanation of Solution
The given reaction is
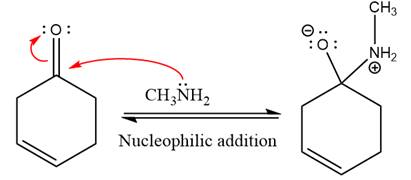
This is an imine formation reaction in which the reaction is catalyzed under mildly acidic condition and excess methylamine acts as the nucleophile.
First three steps are nucleophilic addition reactions on ketone. In the first step, nucleophile
In the next step, deprotonation takes place by the ammonia
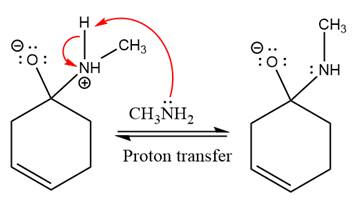
Next, negativively charged oxygen ion abstracts the proton from methylamino ion.
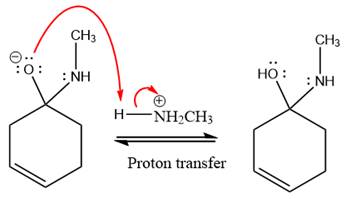
The lone pairs on
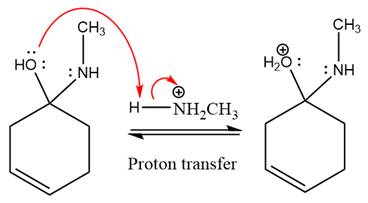
The
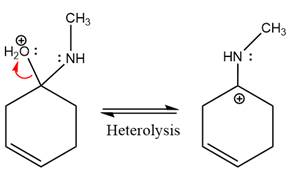
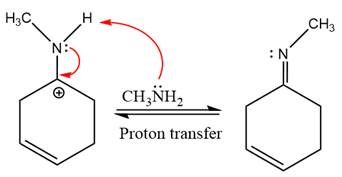
The complete, detailed mechanism of the given reaction under mildly acidic medium is shown below and an imine is the major product.
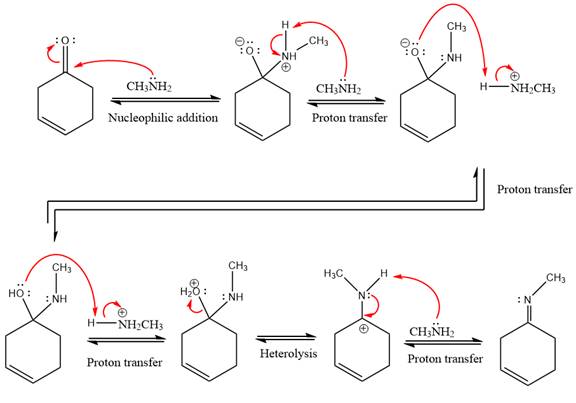
The complete, detailed mechanism of the given reaction under mildly acidic medium and excess methylamine is drawn.
(c)
Interpretation:
The complete, detailed mechanism of a given reaction under acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to ketone or an aldehyde, the uncharged nucleophile ammonia (
Answer to Problem 18.57P
The complete, detailed mechanism of the given reaction in the acidic medium is shown below and an ketone is the major product.
Explanation of Solution
The given reaction is
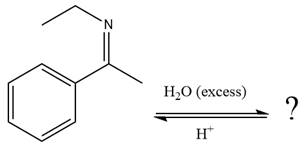
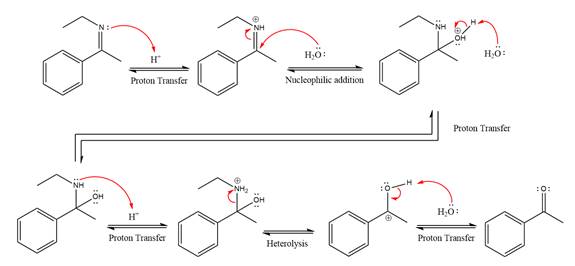
This is an imine hydrolysis reaction under strongly acidic condition to produce ketone as the major product.
In the first step, the
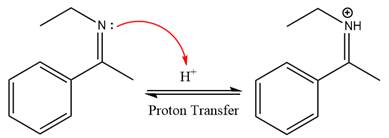
Next, water nucleophile attacks the activated electrophilic carbon by nucleophilic addition reaction.
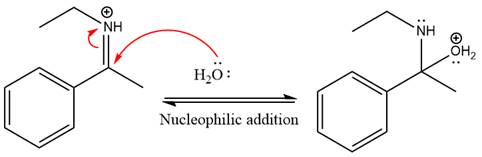
In the next step, deprotonation takes place by the nucleophile produced
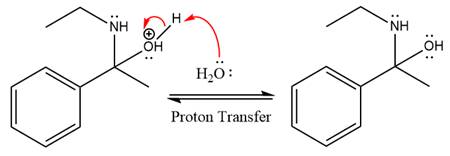
The lone pairs on
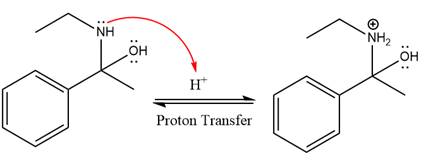
The
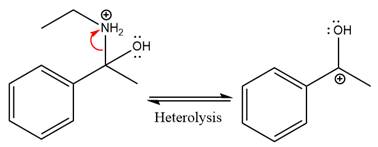
The nucleophile water abstracts the proton in the resonance stabilized carbocation and produces ketone as the major product.
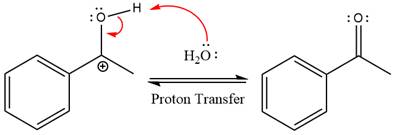
The complete, detailed mechanism of the given reaction under strongly acidic medium is shown below and ketone is the major product.
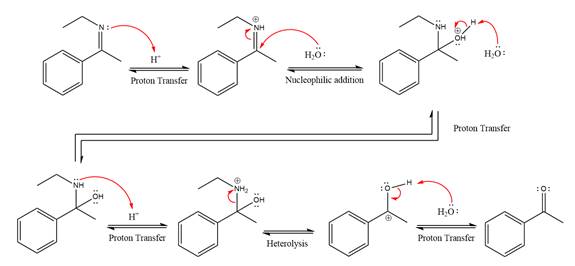
The complete, detailed mechanism of the given reaction under strongly acidic medium and excess water is drawn.
(d)
Interpretation:
The complete, detailed mechanism of the given reaction in the mildly acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to ketone or an aldehyde, the uncharged nucleophile ammonia (
Answer to Problem 18.57P
The complete, detailed mechanism of the given reaction in mildly acidic medium is shown below and enamine is the major product.
Explanation of Solution
The given reaction is
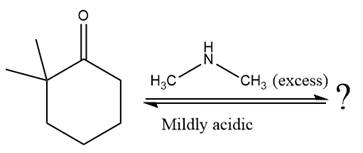
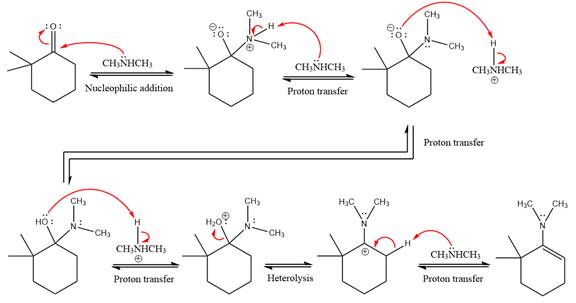
This is an enamine formation reaction in which the reaction is catalyzed under mildly acidic conditions, and the secondary
First three steps are nucleophilic addition reactions on ketone. In the first step, nucleophile
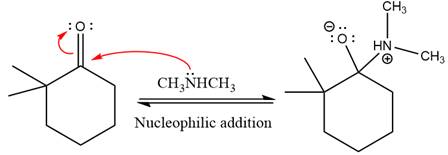
In the next step, deprotonation takes place by
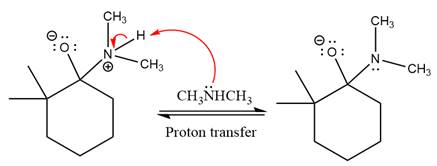
Next, negatively charged oxygen ion abstracts the proton from dimethylamino ion.
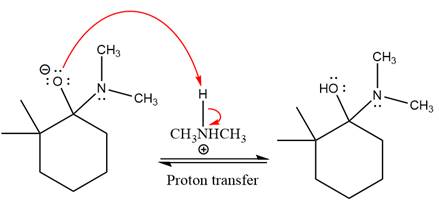
The lone pairs on
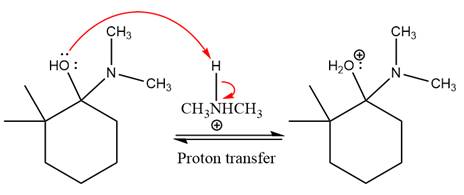
The
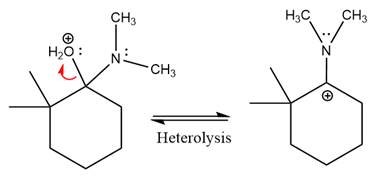
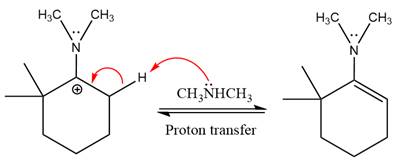
The complete, detailed mechanism of the given reaction in mildly acidic medium is shown below and enamine is the major product.

The complete, detailed mechanism of the given reaction under acidic medium and excess alcohol is drawn.
(e)
Interpretation:
The complete, detailed mechanism of a given reaction under strongly acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to ketone or an aldehyde, the uncharged nucleophile
Answer to Problem 18.57P
The complete, detailed mechanism of the given reaction under strongly acidic medium is shown below and ketone is the major product.
Explanation of Solution
The given reaction is
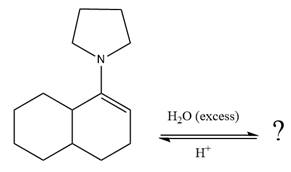
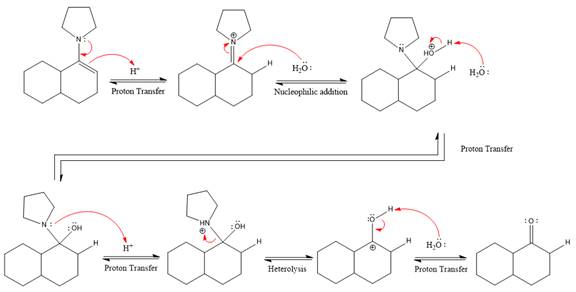
This is an enamine hydrolysis reaction under strongly acidic condition to produce ketone as the major product.
In the first step,
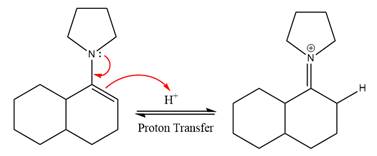
Next, water nucleophile attacks the activated electrophilic carbon by nucleophilic addition reaction.
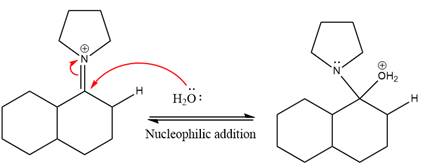
In the next step, deprotonation takes place by the nucleophile produced
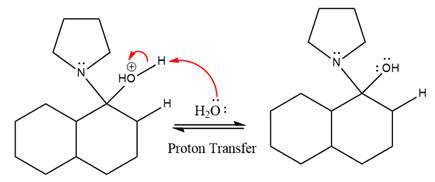
The lone pairs on
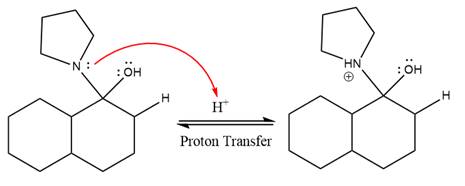
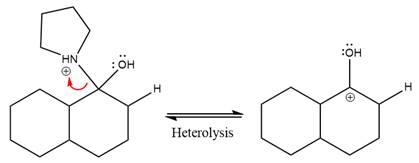
The nucleophile water abstracts the proton in the resonance stabilized carbocation and produces ketone as the major product.
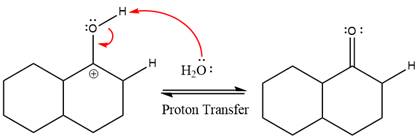
The complete, detailed mechanism of the given reaction in the acidic medium is shown below and an acetal is the major product.
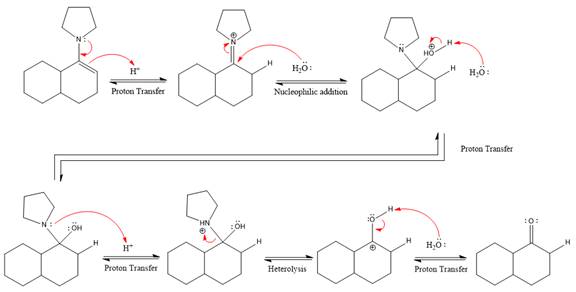
The complete, detailed mechanism of the given reaction under strongly acidic medium and excess water is drawn.
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry: Principles And Mechanisms
- 11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forwardUnder aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardAssign these COSY Spectrumarrow_forward
- Assign these C-NMR and H-NMR Spectrumarrow_forwardPredict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forwardPredict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forward
- Predict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forward
- Predict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





