
(a)
Interpretation:
The reactivity of cyclohexanol and phenol with aqueous
Concept introduction:
The phenol is an aromatic compound having a formula,
Answer to Problem 18.53AP
Phenol is more reactive than cyclohexanol with an aqueous
Explanation of Solution
The sodium hydroxide is a strong base. It abstracts a proton from the stronger acidic compound and forms its sodium salt. In the given case, sodium hydroxide abstracts a proton from phenol and cyclohexanol and form phenoxide ion and cyclohexanol conjugate base respectively. The phenoxide ion gets stabilized by resonance structures, but this is not possible in case of cyclohexanol conjugate base. It is known that more the stability of the conjugate base more the acidity of the compound. In the given case, phenoxide ion is more stable due to this phenoxide is more acidic. Therefore, phenoxide ion is more reactive toward an aqueous
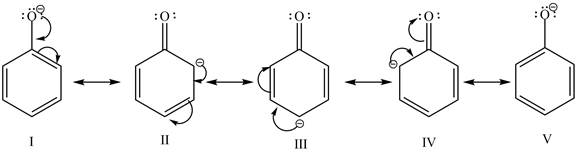
Figure 1
The reactivity of phenol is more as compared to cyclohexanol with an aqueous solution of
(b)
Interpretation:
The reactivity of cyclohexanol and phenol with
Concept introduction:
The phenol is an aromatic compound having a formula,
Answer to Problem 18.53AP
Phenol is more reactive than cyclohexanol with an aqueous
Explanation of Solution
The sodium hydride is a strong base. It abstracts a proton from the stronger acidic compound and forms its sodium salt and hydrogen gas is evolved. In the given case, sodium hydroxide abstracts a proton from phenol and cyclohexanol and form phenoxide ion and cyclohexanol conjugate base respectively. The phenoxide ion gets stabilized by resonance structures, but this is not possible in case of cyclohexanol conjugate base. It is known that more the stability of the conjugate base more the acidity of the compound. In the given case, phenoxide ion is more stable due to this phenoxide is more acidic. Therefore, phenoxide ion is more reactive toward an aqueous
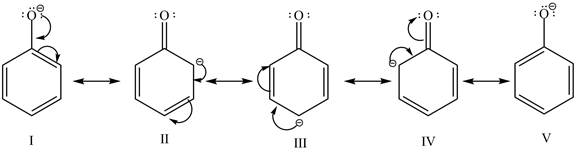
Figure 2
The reactivity of phenol is more as compared to cyclohexanol with an aqueous solution of
(c)
Interpretation:
The reactivity of cyclohexanol and phenol with triflic anhydride in pyridine at
Concept introduction:
The triflic anhydride is a chemical compound which is also known as Trifluoromethanesulfonic anhydride. It has a molecular formula
Answer to Problem 18.53AP
The presence of the more nucleophilic character of cyclohexanol makes it more reactive towards triflic anhydride in pyridine at
Explanation of Solution
In the given case, when triflic acid reacts with cyclohexanol and phenol in the presence of pyridine at
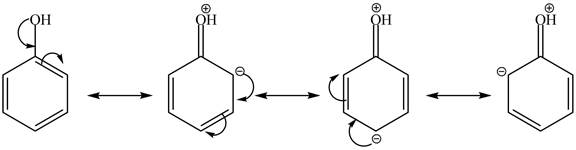
Figure 3
The reactivity of cyclohexanol is more as compared to phenol with triflic acid in pyridine at
(d)
Interpretation:
The reactivity of cyclohexanol and phenol with concentrated aqueous
Concept introduction:
The phenol is an aromatic compound having a formula,
Answer to Problem 18.53AP
The deactivation of the aromatic ring of phenol makes it less reactive toward concentrated aqueous
Explanation of Solution
Cyclohexanol is more nucleophilic as compared to phenol. It is due to the participation of the lone pair of electrons on oxygen in resonance structures of phenol. Therefore, in the given conditions cyclohexanol reacts more rapidly as compared to phenol. When cyclohexanol undergoes protonation in the presence of
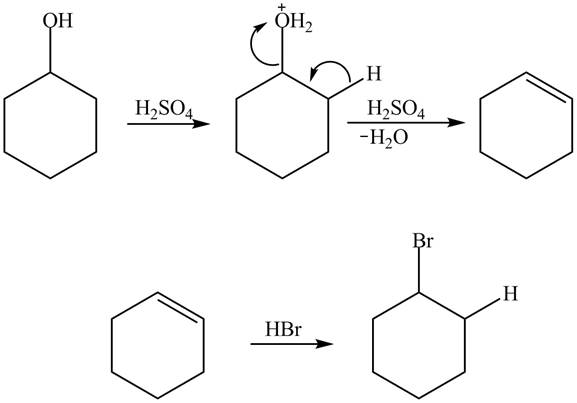
Figure 4
In the case of phenol, it undergoes electrophilic aromatic substitution reaction with
The reactivity of phenol is less with concentrated aqueous
(e)
Interpretation:
The reactivity of cyclohexanol and phenol with
Concept introduction:
The phenol is an aromatic compound having a formula,
Answer to Problem 18.53AP
The phenol is more reactive toward
Explanation of Solution
The phenol is an aromatic compound which shows similar reactions as
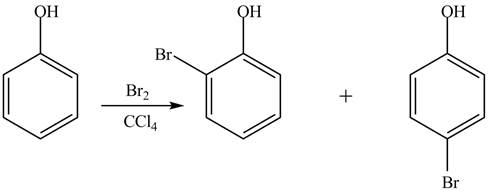
Figure 5
Whereas no such reaction is possible with cyclohexanol because this compound gives addition reaction.
The phenol is an aromatic compound which undergoes electrophilic substitution reaction. Due to this, it is more reactive toward
(f)
Interpretation:
The reactivity of cyclohexanol and phenol with
Concept introduction:
Oxidation is defined as the addition of oxygen atom or removal of the hydrogen atom. The oxidizing agent is the substance that causes oxidation and itself get reduced. The reagent
Answer to Problem 18.53AP
The phenol is an aromatic compound which loses its aromatic character on reaction with
Explanation of Solution
In the given conditions, both the given compounds undergo an oxidation reaction. The reaction of cyclohexanol with
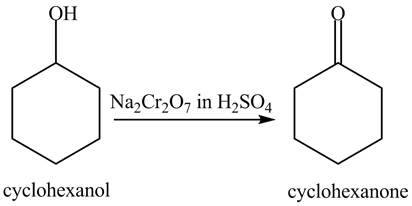
Figure 6
The reaction of phenol with
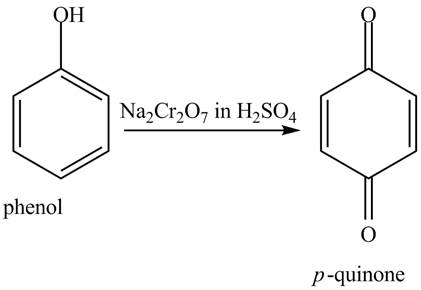
Figure 7
When phenol undergoes oxidation reaction, it loses its aromatic character which means it loses its stability. Therefore, cyclohexanol is more reactive toward
The phenol on reaction with
(g)
Interpretation:
The reactivity of cyclohexanol and phenol with
Concept introduction:
The phenol is an aromatic compound having a formula,
Answer to Problem 18.53AP
The deactivation of the aromatic ring of phenol due to protonation of the hydroxyl group makes it less reactive toward
Explanation of Solution
Cyclohexanol is more nucleophilic as compared to phenol. It is due to the participation of the lone pair of electrons on oxygen in resonance structures of phenol. Therefore, in the given conditions cyclohexanol reacts more rapidly as compared to phenol. When cyclohexanol undergoes protonation in the presence of
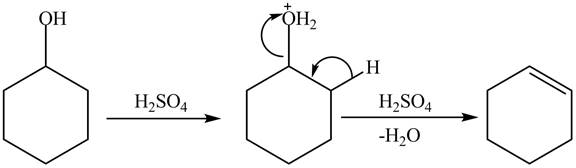
Figure 8
The less nucleophilic character makes phenol less reactive toward
The reactivity of phenol is less with
Want to see more full solutions like this?
Chapter 18 Solutions
EBK ORGANIC CHEMISTRY STUDY GUIDE AND S
- dG = Vdp - SdT + μA dnA + μB dnB + ... so that under constant pressure and temperature conditions, the chemical potential of a component is the rate of change of the Gibbs energy of the system with respect to changing composition, μJ = (∂G / ∂nJ)p,T,n' Using first principles prove that under conditions of constant volume and temperature, the chemical potential is a measure of the partial molar Helmholtz energy (μJ = (∂A / ∂nJ)V,T,n')arrow_forwardThe vapor pressure of dichloromethane at 20.0 °C is 58.0 kPa and its enthalpy of vaporization is 32.7 kJ/mol. Estimate the temperature at which its vapor pressure is 66.0 kPa.arrow_forwardDraw the structure of A, the minor E1 product of the reaction. Cl Skip Part Check F1 esc CH_CH OH, D 3 2 Click and drag to start drawing a structure. 80 R3 F4 F2 F3 @ 2 # $ 4 3 Q W 95 % KO 5 F6 A F7 × G ☐ Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ►II A A F8 F9 F10 FL 6 7 88 & * 8 9 LLI E R T Y U A S D lock LL F G H 0 P J K L Z X C V B N M 9 Harrow_forward
- From the choices given, which two substances have the same crystal structure? (Select both) Group of answer choices ZnS (zincblende) Diamond TiO2 (rutile) ZnS (wurtzite)arrow_forwardPotassium (K) blends with germanium (Ge) to form a Zintl phase with a chemical formula of K4Ge4. Which of the following elements would you expect potassium to blend with to form an alloy? Electronegativities: As (2.0), Cl (3.0), Ge (1.8), K (0.8), S (2.5), Ti (1.5) Group of answer choices Arsenic (As) Sulfur (S) Chlorine (Cl) Titanium (Ti)arrow_forwardConsider two elements, X and Z. Both have cubic-based unit cells with the same edge lengths. X has a bcc unit cell while Z has a fcc unit cell. Which of the following statements is TRUE? Group of answer choices Z has a larger density than X X has more particles in its unit cell than Z does X has a larger density than Z Z has a larger unit cell volume than Xarrow_forward
- How many particles does a face-centered cubic (fcc) unit cell contain? Group of answer choices 2 14 8 4arrow_forwardV Highlight all of the carbon atoms that have at least one beta (B) hydrogen, using red for one ẞ hydrogen, blue for two ẞ hydrogens, and green for three ẞ hydrogens. If none of the carbon atoms have ẞ hydrogens, check the box underneath the molecule. ED X None of the carbon atoms have ẞ hydrogens. Explanation esc 2 Check * F1 F2 1 2 80 # 3 Q W tab A caps lock shift fn control F3 N S option O 694 $ F4 F5 F6 005 % E R D F LL 6 olo 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility A DII F7 F8 87 & * 8 T Y U G H 4 F9 F10 ( 9 0 E F11 F12 உ J K L + || X C V B N M H H command option commandarrow_forwardConsider the reaction below and answer the following questions. Part 1 of 4 Br NaOCH2CH3 Identify the mechanisms involved. Check all that apply. SN 1 SN 2 E1 E2 None of the above Part 2 of 4 Skip Part Check esc F1 F2 lock 1 2 Q W A S #3 80 F3 F4 F5 F6 Save For © 2025 McGraw Hill LLC. All Rights Reserved. Terms ˇˇ % & 4 5 6 89 7 IK A 分 བ F7 F8 F9 F * E R T Y U 8 9 D F G H K V B N M 0 Oarrow_forward
- What kind of holes are not generated when solid-state particles adopt a close packing pattern? Group of answer choices tetrahedral cubic octahedral None of the other choices are correctarrow_forwardFor the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. 田 Major Product: Check ☐ + I Na OH esc F1 F2 2 1 @ 2 Q W tab A caps lock S #3 80 F3 69 4 σ F4 % 95 S Click and drag to sta drawing a structure mm Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use GO DII F5 F6 F7 F8 F9 F10 6 CO 89 & 7 LU E R T Y U 8* 9 0 D F G H J K L Z X C V B N M 36arrow_forwardProblem 7 of 10 Draw the major product of this reaction. Ignore inorganic byproducts. S' S 1. BuLi 2. ethylene oxide (C2H4O) Select to Draw a Submitarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

