
Concept explainers
18-26 Answer true or false.
(a)
(b) Phenols, alcohols, and carboxylic acids have in common the presence of an —OH group.
(c) Carboxylic acids are stronger acids than alcohols but weaker acids than phenols.
(d) The order of acidity of the following carboxylic acids is:
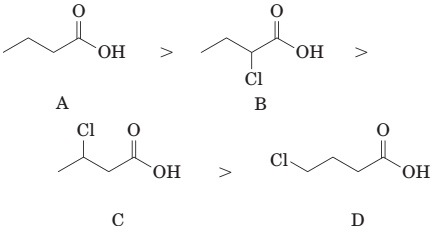
(e) The order of acidity of the following carboxylic acids is:
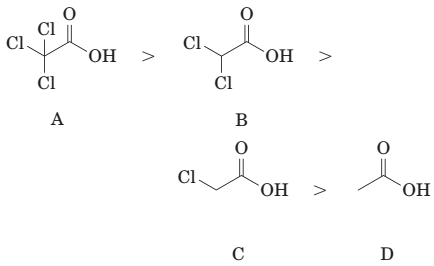
(f) The reaction of benzoic acid with aqueous sodium hydroxide gives sodium benzoate.
(g) A mixture of the following compounds is extracted in order with (1) 1 M HCI, (2) 1 M NaOH, and (3) diethyl ether. Only compound II is extracted into the basic layer.
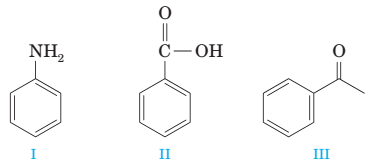
(h) Conversion of compound I to compound II is best accomplished by reduction with NaBH4.
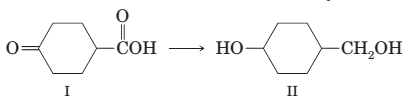
(i) The following ester can be prepared by treating benzoic acid with 1-butanol in the presence of a catalytic amount of H2SO4:
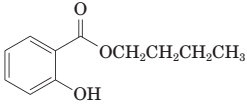
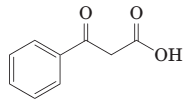
(j) Thermal decarboxylation of this ((-ketoacid gives benzoic acid and carbon dioxide:
(k) Thermal decarboxylation of this (-ketoacid gives 2-pentanone and carbon dioxide.
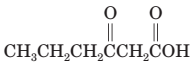
Trending nowThis is a popular solution!

Chapter 18 Solutions
Student Solutions Manual for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th
- the vibrational frequency of I2 is 214.5 cm-1. (i) Using the harmonic oscillator model, evaluate the vibrational partition function and the mean vibrational energy of I2 at 1000K. (ii) What is the characteristic vibrational temperature of I2? (iii) At 1000K, assuming high-temperature approximation, evaluate the vibrational partition function and the mean vibrational energy of I2. (iv) Comparing (i) and (iii), is the high-temperature approximation good for I2 at 1000K?arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardconsider a weak monoprotic acid that is 32 deprotonated at ph 4.00 what is the pka of the weak acidarrow_forward
- How much energy does it take to raise the temperature of 1.0 mol H2O(g) from 100 °C to 200 °C at constant volume? Consider only translational and rotational contributions to the heat capacity. Hint: Use high-temp limit for non-linear molecule when calculating rotational contribution.arrow_forwardwhat was the pH of gastric juice obtained 5.0ml sample of gastric juice taken from a patient several hours after a meal and titrated the juice with 0,2M NaOH t neutrality the neutralization of gastric HCL required 5.0ml NaOH what was the pH of gastric juice?arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- 2. Freckles (F) are dominant to no freckles (f). A heterozygous mother ( father ( have a baby. F = freckles, f= no freckles Genotype Phenotype Possibility 1: Possibility 2: Possibility 3: Possibility 4: and heterozygousarrow_forwardDon't used hand raitingarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





