
Concept explainers
(a)
Interpretation:
The ratio of A- to [HA] with the help of pH and pKa values should be calculated.
Concept Introduction:
The pH of a solution indicates the number of H3 O+ ions in the solution. The mathematical relation between pH and H3 O+ is as given below;.
Acid dissociation constant Ka is related to pKa as;.
Answer to Problem 18.33P
Explanation of Solution
Given Information:
pKa = 5.0.
pH = 2.0.
Calculation:
From pH of solution, concentration of hydroxide ion can be calculated as follows:
Also,
Since,
Thus, by putting the values, the ratio
(b)
Interpretation:
The ratio of A- to [HA] with the help of pH and pKa values should be calculated.
Concept Introduction:
The pH of a solution indicates the number of H3 O+ ions in the solution. The mathematical relation between pH and H3 O+ is as given below;.
Acid dissociation constant Ka is related to pKa as;.
Answer to Problem 18.33P
Given:
pKa = 5.0.
pH = 5.0.
Hence [H3 O+ ] = 10-pH = 10-5.
And Ka = 10-pKa = 10-5.
Since 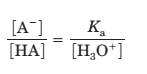
Plug the values of Ka and H3 O+ ions to calculate the ratio of [A- ] to [HA].
[A- ] / [HA] = 10-5 / 10-5 = 1.
Explanation of Solution
Given Information:
pKa = 5.0.
pH = 5.0.
Calculation:
From pH of solution, concentration of hydroxide ion can be calculated as follows:
Also,
Since,
Thus, by putting the values, the ratio
(c)
Interpretation:
The ratio of A- to [HA] with the help of pH and pKa values should be calculated.
Concept Introduction:
The pH of a solution indicates the number of H3 O+ ions in the solution. The mathematical relation between pH and H3 O+ is as given below;.
Acid dissociation constant Ka is related to pKa as;.
Answer to Problem 18.33P
Explanation of Solution
Given Information:
pKa = 5.0.
pH = 7.0.
Calculation:
From pH of solution, concentration of hydroxide ion can be calculated as follows:
Also,
Since,
Thus, by putting the values, the ratio
(d)
Interpretation:
The ratio of A- to [HA] with the help of pH and pKa values should be calculated.
Concept Introduction:
The pH of a solution indicates the number of H3 O+ ions in the solution. The mathematical relation between pH and H3 O+ is as given below;.
Acid dissociation constant Ka is related to pKa as;.
Answer to Problem 18.33P
Explanation of Solution
Given Information:
pKa = 5.0.
pH = 9.0.
Calculation:
From pH of solution, concentration of hydroxide ion can be calculated as follows:
Also,
Since,
Thus, by putting the values, the ratio
(e)
Interpretation:
The ratio of A- to [HA] with the help of pH and pKa values should be calculated.
Concept Introduction:
The pH of a solution indicates the number of H3 O+ ions in the solution. The mathematical relation between pH and H3 O+ is as given below;.
Acid dissociation constant Ka is related to pKa as;.
Answer to Problem 18.33P
Explanation of Solution
Given Information:
pKa = 5.0.
pH = 11.0.
Calculation:
From pH of solution, concentration of hydroxide ion can be calculated as follows:
Also,
Since,
Thus, by putting the values, the ratio
Want to see more full solutions like this?
Chapter 18 Solutions
Student Solutions Manual for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th
- Don't used hand raitingarrow_forwardDon't used Ai solutionarrow_forwardSaved v Question: I've done both of the graphs and generated an equation from excel, I just need help explaining A-B. Below is just the information I used to get the graphs obtain the graph please help. Prepare two graphs, the first with the percent transmission on the vertical axis and concentration on the horizontal axis and the second with absorption on the vertical axis and concentration on the horizontal axis. Solution # Unknown Concentration (mol/L) Transmittance Absorption 9.88x101 635 0.17 1.98x101 47% 0.33 2.95x101 31% 0.51 3.95x10 21% 0.68 4.94x10 14% 24% 0.85 0.62 A.) Give an equation that relates either the % transmission or the absorption to the concentration. Explain how you arrived at your equation. B.) What is the relationship between the percent transmission and the absorption? C.) Determine the concentration of the ironlll) salicylate in the unknown directly from the graph and from the best fit trend-line (least squares analysis) of the graph that yielded a straight…arrow_forward
- Don't used Ai solutionarrow_forwardCalculate the differences between energy levels in J, Einstein's coefficients of estimated absorption and spontaneous emission and life time media for typical electronic transmissions (vnm = 1015 s-1) and vibrations (vnm = 1013 s-1) . Assume that the dipolar transition moments for these transactions are in the order of 1 D.Data: 1D = 3.33564x10-30 C m; epsilon0 = 8.85419x10-12 C2m-1J-1arrow_forwardDon't used Ai solutionarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardIn an induced absorption process:a) the population of the fundamental state is diminishingb) the population of the excited state decreasesc) the non-radiating component is the predominant oned) the emission radiation is consistentarrow_forwardhow a - Cyanostilbenes are made? provide 3 different methods for their synthesisarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


