
Todraw: electron dot diagrams for carbon and hydrogen.
To draw: electron dot diagrams for methane (
Explanation of Solution
Introduction:
Electron dot structures are diagrams demonstrating the bonding of the molecular atoms to the lone pairs of electrons that can occur in the molecule. It is also known as Lewis diagram.
Electron dot diagram gives clear picture that describes the stability of the atom easily which represent the valence electrons around the chemical symbol of the atom.
Atomic number of carbon, hydrogen is 6 and 1 respectively.
Electron dot diagram for carbon, hydrogen and methane are shown below:

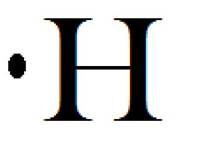
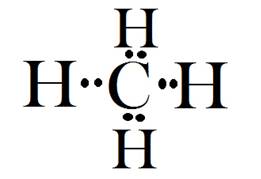
In case of methane, four valance electrons of carbon will pair up with each electron of four hydrogen atoms.
Chapter 18 Solutions
Glencoe Physical Science 2012 Student Edition (Glencoe Science) (McGraw-Hill Education)
Additional Science Textbook Solutions
Campbell Essential Biology with Physiology (5th Edition)
Chemistry (7th Edition)
Microbiology: An Introduction
Organic Chemistry (8th Edition)
Campbell Biology (11th Edition)
Human Anatomy & Physiology (2nd Edition)
- 6. A car drives at steady speed around a perfectly circular track. (a) The car's acceleration is zero. (b) The net force on the car is zero. (c) Both the acceleration and net force on the car point outward. (d) Both the acceleration and net force on the car point inward. (e) If there is no friction, the acceleration is outward.arrow_forward9. A spring has a force constant of 100 N/m and an unstretched length of 0.07 m. One end is attached to a post that is free to rotate in the center of a smooth. table, as shown in the top view in the figure below. The other end is attached to a 1kg disc moving in uniform circular motion on the table, which stretches the spring by 0.03 m. Friction is negligible. What is the centripetal force on the disc? Top View (a) 0.3 N (b) 3.0 N (c) 10 N (d) 300 N (e) 1000 Narrow_forward4. A child has a ball on the end of a cord, and whirls the ball in a vertical circle. Assuming the speed of the ball is constant (an approximation), when would the tension in the cord be greatest? (a) At the top of the circle. (b) At the bottom of the circle. (c) A little after the bottom of the circle when the ball is climbing. (d) A little before the bottom of the circle when the ball is descending quickly. (e) Nowhere; the cord is pulled the same amount at all points.arrow_forward
- 3. In a rotating vertical cylinder (Rotor-ride) a rider finds herself pressed with her back to the rotating wall. Which is the correct free-body diagram for her? (a) (b) (c) (d) (e)arrow_forward8. A roller coaster rounds the bottom of a circular loop at a nearly constant speed. At this point the net force on the coaster cart is (a) zero. (b) directed upward. (c) directed downward. (d) Cannot tell without knowing the exact speed.arrow_forward5. While driving fast around a sharp right turn, you find yourself pressing against the left car door. What is happening? (a) Centrifugal force is pushing you into the door. (b) The door is exerting a rightward force on you. (c) Both of the above. (d) Neither of the above.arrow_forward
- 7. You are flung sideways when your car travels around a sharp curve because (a) you tend to continue moving in a straight line. (b) there is a centrifugal force acting on you. (c) the car exerts an outward force on you. (d) of gravity.arrow_forward1. A 50-N crate sits on a horizontal floor where the coefficient of static friction between the crate and the floor is 0.50. A 20-N force is applied to the crate acting to the right. What is the resulting static friction force acting on the crate? (a) 20 N to the right. (b) 20 N to the left. (c) 25 N to the right. (d) 25 N to the left. (e) None of the above; the crate starts to move.arrow_forward3. The problem that shall not be named. m A (a) A block of mass m = 1 kg, sits on an incline that has an angle 0. Find the coefficient of static friction by analyzing the system at imminent motion. (hint: static friction will equal the maximum value) (b) A block of mass m = 1kg made of a different material, slides down an incline that has an angle 0 = 45 degrees. If the coefficient of kinetic friction increases is μ = 0.5 what is the acceleration of the block? karrow_forward
- 2. Which of the following point towards the center of the circle in uniform circular motion? (a) Acceleration. (b) Velocity, acceleration, net force. (c) Velocity, acceleration. (d) Velocity, net force. (e) Acceleration, net force.arrow_forwardProblem 1. (20 pts) The third and fourth stages of a rocket are coastin in space with a velocity of 18 000 km/h when a smal explosive charge between the stages separate them. Immediately after separation the fourth stag has increased its velocity to v4 = 18 060 km/h. Wha is the corresponding velocity v3 of the third stage At separation the third and fourth stages hav masses of 400 and 200 kg, respectively. 3rd stage 4th stagearrow_forwardMany experts giving wrong answer of this question. please attempt when you 100% sure . Otherwise i will give unhelpful.arrow_forward
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





