
Concept explainers
What ester is formed when butanoic acid
a.
b.
c.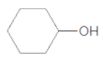
d.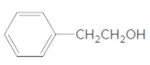
(a)
Interpretation:
The ester formed on treating butanoic acid with CH3OH in the presence of H2SO4 should be determined.
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 55P
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and methanol is:
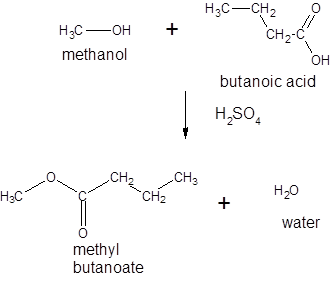
(b)
Interpretation:
The ester formed on treating butanoic acid with CH3CH2CH2OH in the presence of H2SO4 should be determined.
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 55P
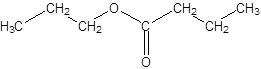
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and propanol is:
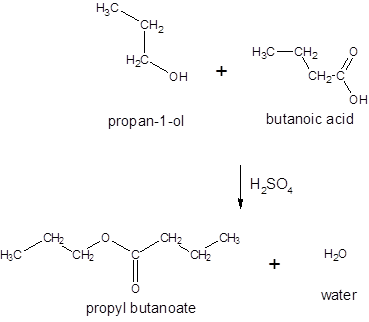
(c)
Interpretation:
The ester formed on treating butanoic acid with following alcohol in the presence of H2SO4 should be determined:
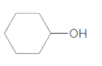
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 55P
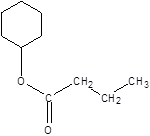
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and cyclohexanol is:
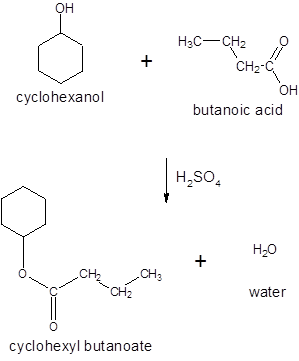
(d)
Interpretation:
The ester formed on treating butanoic acid with following alcohol in the presence of H2SO4 should be determined:
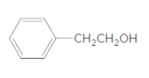
Concept Introduction:
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
Answer to Problem 55P
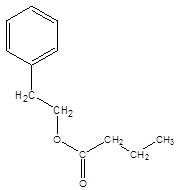
Explanation of Solution
The reaction which results in the formation of at least one ester along with water on heating acids with alcohols is said to be esterification.
The general reaction is written as:
Thus, the reaction between CH3CH2CH2COOH (butanoic acid) and 2-phenylethan-1-ol is:
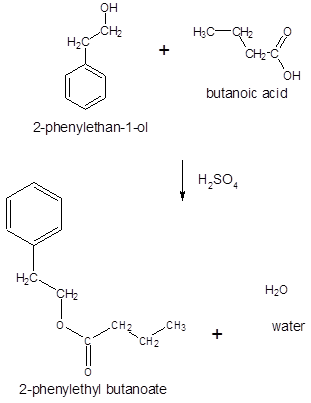
Want to see more full solutions like this?
Chapter 17 Solutions
General, Organic, and Biological Chemistry - 4th edition
- Write the calculate the reaction quotient for the following system, if the partial pressure of all reactantsand products is 0.15 atm: NOCl (g) ⇌ NO (g) + Cl2 (g) H = 20.5 kcalarrow_forwardComplete the spectroscopy with structurearrow_forwardcould you answer the questions and draw the complete mechanismarrow_forward
- Complete the spectroscopy with structurearrow_forwardCalculate the reaction quotient for the reaction:NaOH (s) ⇌ Na+ (aq)+ OH- (aq) + 44.4 kJ [Na+] = 4.22 M [OH-] = 6.41 Marrow_forwardGiven the following concentrations for a system, calculate the value for the reaction quotient: Cl2(g)+ CS2(g) ⇌ CCl4(g)+ S2Cl2(g) Cl2 = 31.1 atm CS2 = 91.2 atm CCl4 = 2.12 atm S2Cl2 = 10.4 atmarrow_forward
- Match each chemical or item with the proper disposal or cleanup mwthod, Not all disposal and cleanup methods will be labeled. Metal sheets C, calcium, choroide solutions part A, damp metal pieces Part B, volumetric flask part A. a.Return to correct lables”drying out breaker. Place used items in the drawer.: Rinse with deionized water, dry as best you can, return to instructor. Return used material to the instructor.: Pour down the sink with planty of running water.: f.Pour into aqueous waste container. g.Places used items in garbage.arrow_forwardWrite the equilibrium constant expression for the following reaction: HNO2(aq) + H2O(l) ⇌ H3O+(aq) + NO2-(aq)arrow_forwardWrite the reaction quotient for: Pb2+(aq) + 2 Cl- (aq) ⇌ PbCl2(s)arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


