
Concept explainers
(a)
The final gauge pressure of the tire.
(a)
Explanation of Solution
Given:
Initial gauge pressure is
Initial temperature of tire is
Final temperature of tire is
The atmospheric pressure is
Formula used:
Write the expression for ideal-
Rearrange the above expression in term of
Here,
Write the expression for gauge pressure.
Here,
Calculation:
Calculate the initial pressure of gas.
Convert the initial temperature from
Convert the final temperature from
Substitute
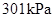 for
for
Substitute
Conclusion:
Thus, the final gauge pressure of tire is
(b)
The final gauge pressure of the tire.
(b)
Explanation of Solution
Given:
Initial gauge pressure is
Initial temperature of tire is
Final temperature of tire is
The atmospheric pressure is
The increase in volume is
Formula used:
Write the expression for ideal-gas law.
The initial and final volume of gas is equal.
Rearrange the above expression in term of
Here,
Write the expression for gauge pressure.
Here,
Calculation:
Calculate the initial pressure of gas.
Convert the initial temperature from
Convert the final temperature from
Calculate the final volume in term of initial volume.
Substitute
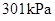 for
for
Substitute
Conclusion:
Thus, the final gauge pressure of tire is
Want to see more full solutions like this?
Chapter 17 Solutions
Physics for Scientists and Engineers
- Consider the circuit shown in the figure below. (Assume L = 5.20 m and R2 = 440 Ω.) (a) When the switch is in position a, for what value of R1 will the circuit have a time constant of 15.4 µs? (b) What is the current in the inductor at the instant the switch is thrown to position b?arrow_forwardCan someone helparrow_forwardCan someone help mearrow_forward
- A particle in a box between x=0 and x=6 has the wavefunction Psi(x)=A sin(2πx). How muchenergy is required for the electron to make a transition to Psi(x)= A’ sin(7π x/3). Draw anapproximate graph for the wavefunction. Find A and A'arrow_forwardA proton is moving with 10^8 m/s speed. Find the De Broglie wavelength associated with theproton and the frequency of that wave.arrow_forwardFind the wavelength of the photon if a (Li--) electron makes a transition from n=4 to n=3. Findthe Bohr radius for each state.arrow_forward
- A photon with wavelength 3000 nm hits a stationary electron. After the collision electron isscattered to 60 degrees. Find the wavelength and frequency of the scattered photon.arrow_forwardA metal has threshold frequency 10^15. Calculate the maximum kinetic energy of the ejectedelectron if a laser beam with wavelength 1.5 10^-7 m is projected on the metal.arrow_forwardDetermine the direction of the vector V, B, or ♬ that is missing from the pair of vectors shown in each scenario. Here, u is the velocity vector of a moving positive charge, B is a constant and uniform magnetic field, and F is the resulting force on the moving charge. 1. 2. 3. B OB F 4. ↑F F 5. 怔 ↑ ↑F Answer Bank 6. ↑ TE Farrow_forward
- Two point charges (+9.80 nC and -9.80 nC) are located 8.00 cm apart. Let U=0 when all of the charges are separated by infinite distances. What is the potential energy if a third point charge q=-4.20 nC is placed at point b? 8.00 cm 8.00 cm 4.00 +4.00 +4.00- cm cm cm HJarrow_forward! Required information Two chloride ions and two sodium ions are in water, the "effective charge" on the chloride ions (CI¯) is −2.00 × 10-21 C and that of the sodium ions (Na+) is +2.00 x 10-21 C. (The effective charge is a way to account for the partial shielding due to nearby water molecules.) Assume that all four ions are coplanar. CT Na+ Na+ 30.0° 45.0% с сг L. where a = 0.300 nm, b = 0.710 nm, and c = 0.620 nm. What is the direction of electric force on the chloride ion in the lower right-hand corner in the diagram? Enter the angle in degrees where positive indicates above the negative x-axis and negative indicates below the positive x-axis.arrow_forwardA pendulum has a 0.4-m-long cord and is given a tangential velocity of 0.2 m/s toward the vertical from a position 0 = 0.3 rad. Part A Determine the equation which describes the angular motion. Express your answer in terms of the variable t. Express coefficients in radians to three significant figures. ΜΕ ΑΣΦ vec (t)=0.3 cos (4.95t) + 0.101 sin (4.95t) Submit Previous Answers Request Answer × Incorrect; Try Again; 6 attempts remainingarrow_forward

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





