
EBK PHYSICS
5th Edition
ISBN: 8220103026918
Author: Walker
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17, Problem 45PCE
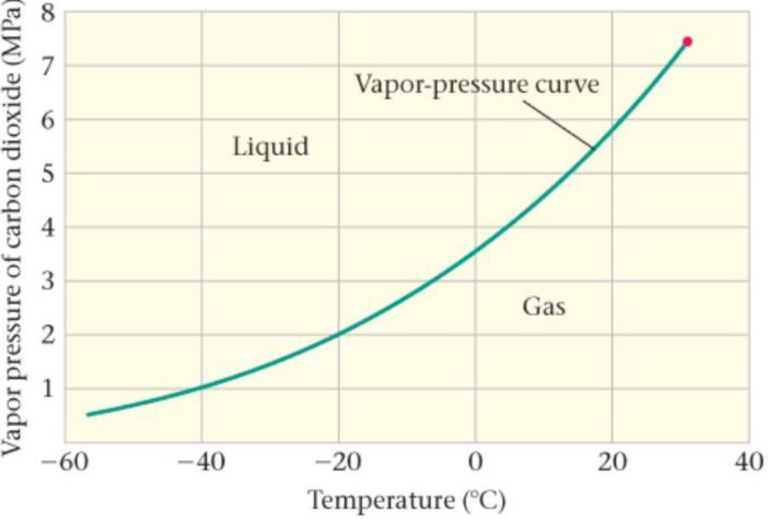
Predict/Calculate The Vapor Pressure of CO2 A portion of the vapor-pressure curve for carbon dioxide is given in Figure 17-36. (a) Estimate the pressure at which CO2 boils at 0 °C. (b) If the temperature is increased, does the boiling pressure increase, decrease, or stay the same? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please don't use Chatgpt will upvote and give handwritten solution
how would i express force in vector form I keep getting a single number
please help me solve this questions. show all calculations and a good graph too :)
Chapter 17 Solutions
EBK PHYSICS
Ch. 17.1 - Rank the following ideal-gas systems in order of...Ch. 17.2 - If the Kelvin temperature of a gas is doubled, by...Ch. 17.3 - A metal rod of a given initial length and...Ch. 17.4 - A portion of a substances phase diagram is shown...Ch. 17.5 - Which requires more heat: melting 100 kg of copper...Ch. 17.6 - An ice cube is placed in a cup of water. A few...Ch. 17 - How is the air pressure in a tightly sealed house...Ch. 17 - The average speed of air molecules in your room is...Ch. 17 - Is it possible to change both the pressure and the...Ch. 17 - Prob. 4CQ
Ch. 17 - A camping stove just barely boils water on a...Ch. 17 - An autoclave is a device used to sterilize medical...Ch. 17 - As the temperature of ice is increased, it changes...Ch. 17 - BIO Isopropyl alcohol is sometimes rubbed onto a...Ch. 17 - A drop of water on a kitchen counter evaporates in...Ch. 17 - (a) Is the number of molecules in one mole of N2...Ch. 17 - Predict/Explain If you put a helium-filled balloon...Ch. 17 - Two containers hold ideal gases at the same...Ch. 17 - Prob. 4PCECh. 17 - BIO After emptying her lungs, a person inhales 4.3...Ch. 17 - An automobile tire has a volume of 0.0185 m3. At a...Ch. 17 - Prob. 7PCECh. 17 - A compressed-air tank holds 0.500 m3 of air at a...Ch. 17 - Four ideal gases have the following pressures, P,...Ch. 17 - A balloon contains 3.9 liters of nitrogen gas at a...Ch. 17 - Prob. 11PCECh. 17 - Predict/Calculate A bicycle tire with a volume of...Ch. 17 - A 515-cm3 flask contains 0.460 g of a gas at a...Ch. 17 - Prob. 14PCECh. 17 - The air inside a hot-air balloon has an average...Ch. 17 - Prob. 16PCECh. 17 - Consider the system described in the previous...Ch. 17 - Prob. 18PCECh. 17 - Prob. 19PCECh. 17 - If the translational speed of molecules in an...Ch. 17 - At what temperature is the rms speed of H2 equal...Ch. 17 - Suppose a planet has an atmosphere of pure ammonia...Ch. 17 - Prob. 23PCECh. 17 - Prob. 24PCECh. 17 - Prob. 25PCECh. 17 - What is the temperature of a gas of CO2 molecules...Ch. 17 - The rms speed of a sample of gas is increased by...Ch. 17 - Prob. 28PCECh. 17 - A 380-mL spherical flask contains 0.065 mol of an...Ch. 17 - Prob. 30PCECh. 17 - A rock climber hangs freely from a nylon rope that...Ch. 17 - BIO To stretch a relaxed biceps muscle 2.5 cm...Ch. 17 - A 22-kg chimpanzee hangs from the end of a...Ch. 17 - The Marianas Trench The deepest place in all the...Ch. 17 - Four cylindrical rods with various cross-sectional...Ch. 17 - Predict/Calculate A steel wire 4.1 m long...Ch. 17 - BIO Spiderweb An orb weaver spider with a mass of...Ch. 17 - Predict/Calculate Two rods of equal length (0.55...Ch. 17 - A piano wire 0.82 m long and 0.93 mm in diameter...Ch. 17 - The formation of ice from water is accompanied by...Ch. 17 - Vapor Pressure for Water Figure 17-35 shows a...Ch. 17 - Using the vapor-pressure curve given in Figure...Ch. 17 - Prob. 43PCECh. 17 - Prob. 44PCECh. 17 - Predict/Calculate The Vapor Pressure of CO2 A...Ch. 17 - Phase Diagram for Water The phase diagram for...Ch. 17 - Phase Diagram for CO2 The phase diagram for CO2 is...Ch. 17 - Prob. 48PCECh. 17 - How much heat must be removed from 1.96 kg of...Ch. 17 - A heat transfer of 9.5 105 J is required to...Ch. 17 - How much heat must be added to 2.55 kg of copper...Ch. 17 - An ammonia refrigeration cycle involves the...Ch. 17 - Prob. 53PCECh. 17 - Prob. 54PCECh. 17 - Prob. 55PCECh. 17 - Figure 17-30 shows a temperature-versus-heat plot...Ch. 17 - Predict/Calculate Suppose the 1.000 kg of water in...Ch. 17 - Prob. 58PCECh. 17 - When you go out to your car one cold winter...Ch. 17 - A large punch bowl holds 3.99 kg of lemonade...Ch. 17 - A 155-g aluminum cylinder is removed from a liquid...Ch. 17 - An 825-g iron block is heated to 352 C and placed...Ch. 17 - Party Planning You are expecting to serve 32 cups...Ch. 17 - Predict/Calculate A 35-g ice cube at 0.0 C is...Ch. 17 - A 48-g block of copper at 12 C is added to 110 g...Ch. 17 - A 0 075-kg ice cube at 0.0 C is dropped into a...Ch. 17 - To help keep her barn warm on cold days, a farmer...Ch. 17 - CE As you go up in attitude, do you expect the...Ch. 17 - Prob. 69GPCh. 17 - Prob. 70GPCh. 17 - Prob. 71GPCh. 17 - Cooling Computers Researchers are developing heat...Ch. 17 - Prob. 73GPCh. 17 - Prob. 74GPCh. 17 - Evaporating Atmosphere Hydrogen gas evaporates...Ch. 17 - Prob. 76GPCh. 17 - A Boiling Geyser (a) The column of water that...Ch. 17 - A Melting Glacier (a) A glacier is made of ice of...Ch. 17 - Peter catches a 4 2-kg striped bass on a fishing...Ch. 17 - A steel ball (density=7860kg/m3) with a diameter...Ch. 17 - A lead brick with the dimensions shown in Figure...Ch. 17 - (a) Find the amount of heat that must be extracted...Ch. 17 - Mighty Ice Lift A tremendous force is generated...Ch. 17 - Orthopedic Implants Metals such as titanium and...Ch. 17 - Students on a spring break picnic bring a cooler...Ch. 17 - A 5.9-kg block of ice at 1.5 C slides on a...Ch. 17 - A cylindrical copper rod 37 cm long and 7.5 cm in...Ch. 17 - Prob. 88PPCh. 17 - Prob. 89PPCh. 17 - Prob. 90PPCh. 17 - Prob. 91PPCh. 17 - Referring to Example 17-17 (a) Find the final...Ch. 17 - Referring to Example 17-17 (a) Find the final...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Define and discuss these terms: (a) synapsis, (b) bivalents, (c) chiasmata, (d) crossing over, (e) chromomeres,...
Concepts of Genetics (12th Edition)
Explain how you can determine whether fault N is older or younger than igneous intrusion J.
Applications and Investigations in Earth Science (9th Edition)
What global policy changes and what individual choices can help us sustain the planet that sustains us?
Biology: Life on Earth with Physiology (11th Edition)
Match the following cell types with their correct definition. _________Macrophage _________NK cell _________Eos...
Human Anatomy & Physiology (2nd Edition)
15. A good scientific hypothesis is based on existing evidence and leads to testable predictions. What hypothes...
Campbell Biology: Concepts & Connections (9th Edition)
8. A human maintaining a vegan diet (containing no animal products) would be a:
a. producer
b. primary consume...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- What is the force (in N) on the 2.0 μC charge placed at the center of the square shown below? (Express your answer in vector form.) 5.0 με 4.0 με 2.0 με + 1.0 m 1.0 m -40 με 2.0 μCarrow_forwardWhat is the force (in N) on the 5.4 µC charge shown below? (Express your answer in vector form.) −3.1 µC5.4 µC9.2 µC6.4 µCarrow_forwardAn ideal gas in a sealed container starts out at a pressure of 8900 N/m2 and a volume of 5.7 m3. If the gas expands to a volume of 6.3 m3 while the pressure is held constant (still at 8900 N/m2), how much work is done by the gas? Give your answer as the number of Joules.arrow_forward
- The outside temperature is 25 °C. A heat engine operates in the environment (Tc = 25 °C) at 50% efficiency. How hot does it need to get the high temperature up to in Celsius?arrow_forwardGas is compressed in a cylinder creating 31 Joules of work on the gas during the isothermal process. How much heat flows from the gas into the cylinder in Joules?arrow_forwardThe heat engine gives 1100 Joules of energy of high temperature from the burning gasoline by exhausting 750 Joules to low-temperature . What is the efficiency of this heat engine in a percentage?arrow_forward
- L₁ D₁ L₂ D2 Aluminum has a resistivity of p = 2.65 × 10 8 2. m. An aluminum wire is L = 2.00 m long and has a circular cross section that is not constant. The diameter of the wire is D₁ = 0.17 mm for a length of L₁ = 0.500 m and a diameter of D2 = 0.24 mm for the rest of the length. a) What is the resistance of this wire? R = Hint A potential difference of AV = 1.40 V is applied across the wire. b) What is the magnitude of the current density in the thin part of the wire? Hint J1 = c) What is the magnitude of the current density in the thick part of the wire? J₂ = d) What is the magnitude of the electric field in the thin part of the wire? E1 = Hint e) What is the magnitude of the electric field in the thick part of the wire? E2 =arrow_forwardplease helparrow_forwardA cheetah spots a gazelle in the distance and begins to sprint from rest, accelerating uniformly at a rate of 8.00 m/s^2 for 5 seconds. After 5 seconds, the cheetah sees that the gazelle has escaped to safety, so it begins to decelerate uniformly at 6.00 m/s^2 until it comes to a stop.arrow_forward
- A projectile is fired with an initial speed of 40.2 m/s at an angle of 35.0 degree above the horizontal on a long flat firing range. Determine. please help and show work for them so i can understand.arrow_forwardpls helparrow_forwardJ K L The graph in the figure shows the position of an object as a function of time. The letters H-L represent particular moments of time. At which moments shown (H, I, etc.) is the speed of the object the greatest? + Position H I K Timearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY