
Introduction to General, Organic and Biochemistry
12th Edition
ISBN: 9780357119303
Author: Bettelheim, Frederick A., Brown, William H., Campbell, Mary K., FARRELL, Shawn O., Torres, Omar
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 17, Problem 44P
18-47 Methylparaben and propylparaben are used as preservatives in foods, beverages, and cosmetics.
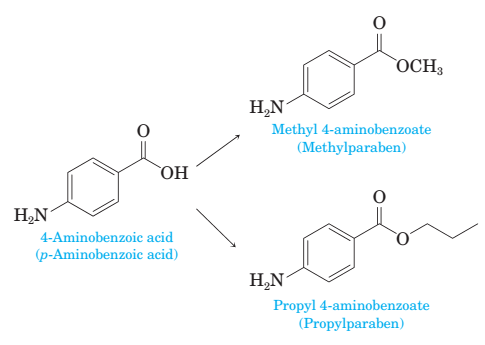
Show how each of these preservatives can be prepared from 4-aminobenzoic acid.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
7. Use Pauling's electronegativity values (Table 1.7) and Ketelaar triangle (Fig. 2.28) to classify
bonding in:
(3 points)
a) CIF3
b) ZnCl2
c) PbS
7. What is the IUPAC name of the following compound?
A) (R)-1-oxo-2-butanol
C) (R)-2-hydroxybutanal
E) (S)-1-formyl-1-propanol
B) (S)-1-oxo-2-butanol
D) (S)-2-hydroxybutanal
OH
H
Cual es la formula semidesarrollada del 3-metil-1-butino?
Chapter 17 Solutions
Introduction to General, Organic and Biochemistry
Ch. 17.2 - Prob. 17.1QCCh. 17.5 - Prob. 17.2QCCh. 17.5 - Prob. 17.3QCCh. 17 - 18-4 Answer true or false. (a) The functional...Ch. 17 - Prob. 2PCh. 17 - 18-6 Name and draw structural formulas for the...Ch. 17 - 18-7 Write the IUPAC name for each carboxylic...Ch. 17 - 18-8 Write the IUPAC name for each carboxylic...Ch. 17 - Prob. 6PCh. 17 - Prob. 7P
Ch. 17 - Prob. 8PCh. 17 - Prob. 9PCh. 17 - Prob. 10PCh. 17 - 18-14 Answer true or false. (a) Carboxylic acids...Ch. 17 - 18-15 Draw a structural formula for the dimer...Ch. 17 - 18-16 Propanedioic (malonic) acid forms an...Ch. 17 - 18-17 Hexanoic (caproic) acid has a solubility in...Ch. 17 - 18-18 Propanoic acid and methyl acetate are...Ch. 17 - 18-19 The following compounds have approximately...Ch. 17 - Prob. 17PCh. 17 - Prob. 18PCh. 17 - Prob. 19PCh. 17 - 18-23 Characterize the structural features...Ch. 17 - Prob. 21PCh. 17 - Prob. 22PCh. 17 - 18-26 Answer true or false. (a) Carboxylic acids...Ch. 17 - Prob. 24PCh. 17 - 18-28 Arrange these compounds in order of...Ch. 17 - 18-29 Complete the equations for these acid—base...Ch. 17 - 18-30 Complete the equations for these acid-base...Ch. 17 - 18-31 Formic acid is one of the components...Ch. 17 - Prob. 29PCh. 17 - Prob. 30PCh. 17 - Prob. 31PCh. 17 - Prob. 32PCh. 17 - Prob. 33PCh. 17 - Prob. 34PCh. 17 - 18-38 Which is the stronger base: CH3CH2NH2 or...Ch. 17 - Prob. 36PCh. 17 - Prob. 37PCh. 17 - 18-41 Complete these examples of Fischer...Ch. 17 - Prob. 39PCh. 17 - Prob. 40PCh. 17 - Prob. 41PCh. 17 - Prob. 42PCh. 17 - 18-46 Procaine (its hydrochloride salt is marketed...Ch. 17 - 18-47 Methylparaben and propylparaben are used as...Ch. 17 - 18-48 4-Aminobenzoic acid is prepared from benzoic...Ch. 17 - Prob. 46PCh. 17 - Prob. 47PCh. 17 - Prob. 48PCh. 17 - Prob. 49PCh. 17 - Prob. 50PCh. 17 - Prob. 51PCh. 17 - Prob. 52P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. A graph shown below shows first ionization energies for elements from H to Ne. First ionization energy/kJ mol 2500 2000 1500 1000 500 T T T T 1 2 3 5 6 7 8 9 10 Atomic number a) Using arguments of electronic structure, explain why ionization energy of Li is much lower than that of H. (2 points) then dips at O. b) Using the same arguments, explain why ionization energy increases from B to N, and (3 points)arrow_forwardGive the name of this compound, including stereochemistry if relevant: CICH2 CH3 Br CH₂CH=CH2 Write in the product, including stereochemistry where relevant, for these reactions. See end of ch. 8, p. 301-303. 1. 03 a) 2-methyl-2-pentene -> 2. Zn, H* Br2 b) 1-ethylcyclopentene -->arrow_forwardNonearrow_forward
- 3. You may want to read paragraph 1.5 in your textbook before answering this question. Give electron configuration (short-hand notation is fine) for: (5 points) 3+ a) Manganese atom and Mn³+ b) Se atom c) Cu atom and Cu+arrow_forwardPlease correct answer and don't use hand ratingarrow_forwardNonearrow_forward
- Nonearrow_forwardHowever, why are intermolecular forces in metallic and ionic compounds not discussed as extensively? Additionally, what specific types of intermolecular attractions exist in metals and ionic compoundsarrow_forwardWhat is the preparation of 1 Liter of 0.1M NH4Cl buffer at pH 9.0 with solid NH4Cl and 0.1M NaOH. How would I calculate the math to describe this preparation? How would I use Henderson-Hasselbach equation?arrow_forward
- C Predict the major products of this organic reaction. Be sure you use wedge and dash bonds when necessary, for example to distinguish between major products with different stereochemistry. : ☐ + x G C RCO₂H Click and drag to start drawing a structure.arrow_forwardFill in the blanks by selecting the appropriate term from below: For a process that is non-spontaneous and that favors products at equilibrium, we know that a) ΔrG∘ΔrG∘ _________, b) ΔunivSΔunivS _________, c) ΔsysSΔsysS _________, and d) ΔrH∘ΔrH∘ _________.arrow_forwardHighest occupied molecular orbital Lowest unoccupied molecular orbital Label all nodes and regions of highest and lowest electron density for both orbitals.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY