
Principles of Physics: A Calculus-Based Text, Hybrid (with Enhanced WebAssign Printed Access Card)
5th Edition
ISBN: 9781305586871
Author: Raymond A. Serway, John W. Jewett
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 17, Problem 27P
A
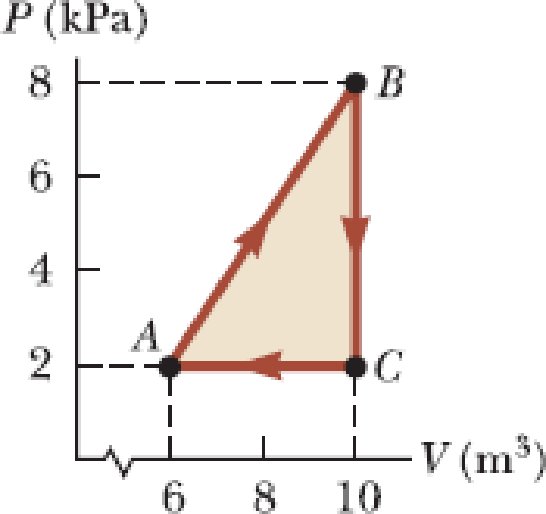
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
look at answer show all work step by step
Look at the answer and please show all work step by step
3. As a woman, who's eyes are h = 1.5 m above the ground, looks down the road sees a tree with height
H = 9.0 m. Below the tree is what appears to be a reflection of the tree. The observation of this apparent
reflection gives the illusion of water on the roadway. This effect is commonly called a mirage. Use the results of questions 1 and 2 and the principle of ray reversibility to analyze the diagram below. Assume that light leaving the top of the tree bends toward the horizontal until it just grazes ground level. After that, the ray bends upward eventually reaching the woman's eyes. The woman interprets this incoming light as if it came from an image of the tree. Determine the size, H', of the image. (Answer 8.8 m)
please show all work step by step
Chapter 17 Solutions
Principles of Physics: A Calculus-Based Text, Hybrid (with Enhanced WebAssign Printed Access Card)
Ch. 17.2 - Prob. 17.1QQCh. 17.3 - Prob. 17.2QQCh. 17.3 - Prob. 17.3QQCh. 17.5 - Prob. 17.4QQCh. 17.6 - Characterize the paths in Figure 17.10 as...Ch. 17.7 - (i) How does the internal energy of an ideal gas...Ch. 17.10 - Prob. 17.7QQCh. 17 - Prob. 1OQCh. 17 - A 100-g piece of copper, initially at 95.0C, is...Ch. 17 - Prob. 3OQ
Ch. 17 - Prob. 4OQCh. 17 - Prob. 5OQCh. 17 - Prob. 6OQCh. 17 - Prob. 7OQCh. 17 - Prob. 8OQCh. 17 - Prob. 9OQCh. 17 - Prob. 10OQCh. 17 - Star A has twice the radius and twice the absolute...Ch. 17 - If a gas is compressed isothermally, which of the...Ch. 17 - When a gas undergoes an adiabatic expansion, which...Ch. 17 - Ethyl alcohol has about one-half the specific heat...Ch. 17 - Prob. 15OQCh. 17 - Prob. 1CQCh. 17 - Prob. 2CQCh. 17 - Pioneers stored fruits and vegetables in...Ch. 17 - Why is a person able to remove a piece of dry...Ch. 17 - Prob. 5CQCh. 17 - Prob. 6CQCh. 17 - It is the morning of a day that will become hot....Ch. 17 - You need to pick up a very hot cooking pot in your...Ch. 17 - Rub the palm of your hand on a metal surface for...Ch. 17 - Prob. 10CQCh. 17 - Prob. 11CQCh. 17 - Prob. 12CQCh. 17 - On his honeymoon, James Joule traveled from...Ch. 17 - Consider Joules apparatus described in Figure...Ch. 17 - Prob. 3PCh. 17 - Prob. 4PCh. 17 - Prob. 5PCh. 17 - Prob. 6PCh. 17 - Prob. 7PCh. 17 - Prob. 8PCh. 17 - Prob. 9PCh. 17 - Prob. 10PCh. 17 - Prob. 11PCh. 17 - Prob. 12PCh. 17 - Prob. 13PCh. 17 - Prob. 14PCh. 17 - In an insulated vessel, 250 g of ice at 0C is...Ch. 17 - Prob. 16PCh. 17 - Prob. 17PCh. 17 - Prob. 18PCh. 17 - A 1.00-kg block of copper at 20.0C is dropped into...Ch. 17 - A resting adult of average size converts chemical...Ch. 17 - Prob. 21PCh. 17 - Prob. 22PCh. 17 - An ideal gas is enclosed in a cylinder with a...Ch. 17 - Prob. 24PCh. 17 - Prob. 25PCh. 17 - A sample of an ideal gas goes through the process...Ch. 17 - A thermodynamic system undergoes a process in...Ch. 17 - A gas is taken through the cyclic process...Ch. 17 - Consider the cyclic process depicted in Figure...Ch. 17 - Why is the following situation impossible? An...Ch. 17 - An ideal gas initially at 300 K undergoes an...Ch. 17 - In Figure P17.32, the change in internal energy of...Ch. 17 - Prob. 33PCh. 17 - Prob. 34PCh. 17 - Prob. 35PCh. 17 - Prob. 36PCh. 17 - Prob. 37PCh. 17 - One mole of an ideal gas does 3 000 J of work on...Ch. 17 - A 1.00-mol sample of hydrogen gas is heated at...Ch. 17 - A sample of a diatomic ideal gas has pressure P...Ch. 17 - Prob. 41PCh. 17 - Prob. 42PCh. 17 - Prob. 43PCh. 17 - Review. This problem is a continuation of Problem...Ch. 17 - Prob. 45PCh. 17 - A 2.00-mol sample of a diatomic ideal gas expands...Ch. 17 - Prob. 47PCh. 17 - An ideal gas with specific heat ratio confined to...Ch. 17 - Prob. 49PCh. 17 - Prob. 50PCh. 17 - Prob. 51PCh. 17 - Prob. 52PCh. 17 - Air (a diatomic ideal gas) at 27.0C and...Ch. 17 - Prob. 54PCh. 17 - Prob. 55PCh. 17 - Prob. 56PCh. 17 - Prob. 57PCh. 17 - Prob. 58PCh. 17 - Prob. 59PCh. 17 - Prob. 60PCh. 17 - Prob. 61PCh. 17 - Prob. 62PCh. 17 - The surface of the Sun has a temperature of about...Ch. 17 - Prob. 64PCh. 17 - At high noon, the Sun delivers 1 000 W to each...Ch. 17 - A theoretical atmospheric lapse rate. Section 16.7...Ch. 17 - Prob. 67PCh. 17 - A sample of a monatomic ideal gas occupies 5.00 L...Ch. 17 - An aluminum rod 0.500 m in length and with a...Ch. 17 - Prob. 70PCh. 17 - Prob. 71PCh. 17 - Prob. 72PCh. 17 - Prob. 73PCh. 17 - Prob. 74PCh. 17 - Prob. 75PCh. 17 - Prob. 76PCh. 17 - Prob. 77PCh. 17 - Prob. 78PCh. 17 - Prob. 79PCh. 17 - Prob. 81PCh. 17 - Prob. 82PCh. 17 - Prob. 84PCh. 17 - Prob. 85PCh. 17 - Prob. 86PCh. 17 - Prob. 87PCh. 17 - Prob. 88PCh. 17 - Water in an electric teakettle is boiling. The...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A piece of silicon semiconductor has length L=0.01cm and cross-section in a square shape with an area of A=5×10−4cm2 . The semiconductor is doped with 1012cm−3 Phosphorus atoms and 1017cm−3 Boron atoms. An external electric field E=1.5×104N/C is applied to the silicon piece along the length direction, through the cross section. What is the total current in the silicon at T=300K? Assume the mobility of silicon is 1400cm2V−1s−1 for electrons and 450cm2V−1s−1 for holes, respectively. Assume the intrinsic carrier concentration in silicon is 1010cm−3 . Give your answer in mA, rounded to 3 significant figures. Just enter the number, nothing else.arrow_forwardAn impurity with a charge of 2e is placed in a three-dimensional metal. Assume that the Friedel sum rule holds for this system, and only the scattering phase shifts from the electrons contribute to this sum (we don't need to consider ion phase shifts). This metal has a spherical Fermi surface with Fermi wave vector kF . The only degeneracy for the electrons at the Fermi surface is spin (two-fold) and angular momentum ( 2l+1 for each angular momentum l ). Ignore scattering for l>2 and assume that the scattering doesn't depend on the spin degree of freedom. Denote the scattering phase shift at the Fermi wave vector in the l -th angular momentum channel as δl(kF) . If δ0(kF)=11π31 , and δ1(kF)=π29 , what is δ2(kF)? Round your answer to three significant figures. Just enter the number, nothing else.arrow_forwardA pilot with a mass of 75 kg is flying an airplane at a true airspeed of 55m/s in air that is still relative to the ground. The pilot enters a coordinated turn of constant bank angle and constant altitude, and the pilot experiences an effective weight of 1471.5N normal to the wings of the plane. What is the rate of turn (in degrees per second) for the aircraft? Round your answer to three significant figures. Just enter the number, nothing else.arrow_forward
- Imagine you are out for a stroll on a sunny day when you encounter a lake. Unpolarized light from the sun is reflected off the lake into your eyes. However, you notice when you put on your vertically polarized sunglasses, the light reflected off the lake no longer reaches your eyes. What is the angle between the unpolarized light and the surface of the water, in degrees, measured from the horizontal? You may assume the index of refraction of air is nair=1 and the index of refraction of water is nwater=1.33 . Round your answer to three significant figures. Just enter the number, nothing else.arrow_forwardRed, yellow, green, and blue light with wavelengths of λred=700 nm , λyellow=580 nm , λgreen=520 nm , and λblue=475 nm are directed at a slit that is 20 μm wide at normal incidence. The light hits a screen 1 m behind the slit. Which color of light will have an interference minimum closest to a point 10 cm away from its central maxima? You may assume the small angle approximation sinθ≈tanθ≈θ for angles smaller than 10∘ . Just enter the wavelength of that color in nm, nothing else.arrow_forwardIn the circuit shown, the switch is initially open and the capacitor isuncharged. What will be the current through R1 the instant after the switch isclosed? Take V=10 V, R1 = 20 W, R2 = 20 W, R3 = 10 W and C = 2 mF.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College
Thermodynamics: Crash Course Physics #23; Author: Crash Course;https://www.youtube.com/watch?v=4i1MUWJoI0U;License: Standard YouTube License, CC-BY